Designed to be Different from Day 1 - BD
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
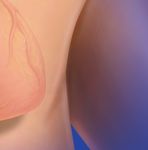
Side-Hole Free:
Designed to be Different
from Initial Placement
· The Pristine™ Long-Term Hemodialysis Catheter is designed to support
anterior, posterior tip placement orientation in the mid right atrium
· The notched tip of the Pristine™ Catheter is designed to help resist
positional occlusion
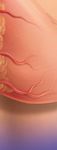
Unique Y-Tip™
Distal Lumen Design
· Side-hole free tip is designed to help
facilitate blood clot aspiration prior to
hemodialysis treatment
· Side-hole free tip designed to help
minimize thrombus adhesion that can
be associated with side-hole catheters
· Demonstrated low recirculation rates
in both forward and reverse1
1
Tested using 55 cm tip-to-cuff length Pristine™ Long-Term Hemodialysis Catheters (n=37). Recirculation test performed using 5% saline as blood simulant at a blood flow rate of ~5 liters/minute and catheter flow rate of 350
mL/min. The mean recirculation rates were 2.7% in forward flow and 2.8% in reverse flow. Bench data on file, Becton, Dickinson and Company, Tempe, AZ. Bench data may not necessarily correlate to clinical performance.
Different test methods may yield different results.CLINICAL EXPERIENCE B EN C H T E S T DATA
Intervention-Free at 60 Days Flow Rates, Pressures & Recirculation
Adequate hemodialysis (flow >300 mL/min) without need for additional Flow Rates and Pressures
interventions to maintain catheter patency to maintain flow or correct device failures Pristine™ Long-Term Hemodialysis Catheters demonstrated high flow rates at low pressures.
In a prospective, single-center, non-randomized, open-label feasibility study performed in the
Dominican Republic, 45 patients who received the 15.5F Pristine™ Long-Term Hemodialysis Catheter Flow Rates1
were followed for 6 months post-catheter implantation. All catheters were patent at 30 days post
implantation. Primary patency at 60- and 180-days post procedure was 100% and 91%, respectively.
This was a prospective analysis without prespecified, hypothesis-tested endpoints. Data are
descriptive only. The study was conducted on subjects with End Stage Renal Disease or Acute Renal 19 cm - 33 cm Catheter Lengths 500 ml/min
Failure outside of the United States where clinical practices may differ and results may or may not be
representative of patients in the United States. 1
55 cm Catheter Length 379 ml/min
Primary Patency Through 6 Months
100 0 100 200 300 400 500
Flow Rate (ml/min)
Flow rates at an arterial maximum pressure of -250 mmHg or less
80
Percent Patent
60
100%
PATENCY
100%
PATENCY
91%
PATENCY
OBSERVED OBSERVED OBSERVED
AT 30 DAYS
40 AT 60 DAYS AT 180 DAYS
Recirculation
PRIMARY
END POINT
20
The Pristine™ Catheter’s
Symmetric Y-Tip™ distal
0 lumens are designed to
0 30 60 90 120 150 180 help minimize recirculation.2
Follow-up (Days)
Pristine™ Catheters observed 100% patency at 30 and 60 days,
completely intervention-free, while 91% of Pristine™ Catheters
were intervention-free at 180 days.
1
Tested using 19 cm (n=38); 23 cm (n=40); 28 cm (n=38); 33 cm (n=40) and 55 cm (n=39) tip-to-cuff length Pristine™ Long-Term Hemodialysis Catheters. Flow test performed using glycerin: water solution with a
viscosity of 3.2-3.7 cP at 36-38° C. At an arterial maximum pressure of -250 mmHg or less, the maximum flow rates observed were 500 mL/min (19 cm through 33 cm tip-to-cuff lengths) and 379 mL/min (55 cm
tip-to-cuff length). In order to show the the flow rate at -250 mmHg pressure limit, the linear curve fit for the negative side was used to find the flow rate corresponding to indicated pressure, then the flow rate
value was used to estimate the positive venous pressure. Bench data on file. Bench data may not necessarily correlate to clinical performance. Different test methods may yield different results.
2
Tested using 55 cm tip-to-cuff length Pristine™ Long-Term Hemodialysis Catheters (n=37). Recirculation test performed using 5% saline as blood simulant at a blood flow rate of ~5 liters/minute and catheter flow
rate of 350 mL/min. The mean recirculation rates were 2.7% in forward flow and 2.8% in reverse flow. Bench data on file, Becton, Dickinson and Company, Tempe, AZ. Bench data may not necessarily correlate to
1
Data on File, Becton, Dickinson and Company, Tempe, AZ. clinical performance. Different test methods may yield different results.B EN C H T E S T DATA
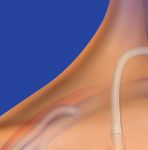
Designed for Accurate Placement
Kink & Positional Occlusion Using Familiar Components
Kink Diameter
Fixed Suture Wings
The Pristine™ Catheter shaft demonstrated small · Promote and enable
0.66"
kink diameter.1 Kink Diameter is defined as the AVERAGE KINK stability
DIAMETER1
furtherest distance achieved before a kink occurs.
2
Tissue In-Growth Cuff
Flow-Pressure in Positional Occlusion Test Environment · Helps to secure device
Pristine™ Catheters demonstrated low arterial and venous pressures in place
Average Pressure2
Polyurethane Shaft
· Strength for longevity
-217 Forward Forward 186 · Softness for flexibility
AirGuard™ Valved
and patient comfort
Introducer
-217 Reverse Reverse 184 · Helps reduce risk of
air embolism
-250 -200 -150 -100 -50 0 50 100 150 200 250
Arterial Average Pressure (mmHg) Venous Average Pressure (mmHg)
Tested using 33 cm length catheters. n=40
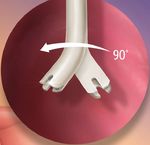
Notched Y-Tip™ Distal Lumen Design
In a simulated flow occlution test, the Pristine™ Catheter
demonstrated low average venous and arterial
pressures with none exceeding ±250 mmHg2 Unique Y-Tip™ StruXure™ Guidewire
Distal Lumen Design · Designed with enhanced
kink resistance compared
1
Tested using shafts from 55 cm tip to cuff straight catheters (n=39). Bench data on file, Becton, Dickinson and Company, Tempe, AZ. Bench data may not necessarily correlate to clinical performance. Different test methods
to stainless steel
may yield different results.
2
In simulated flow occlusion testing at 300 mL/min, the Pristine™ Long-Term Hemodialysis Catheter (33 cm length, n=40) demonstrated average venous and arterial pressures of 186 mmHg and -217 mmHg in forward and
184 mmHg and -217 mmHg in reverse, respectively. Bench data on file, Becton, Dickinson and Company, Tempe, AZ. Bench data may not necessarily correlate to clinical performance. Different test methods may yield
different results.Tip to Cuff Tip to Hub Product Codes Standard Kit Contents
Length (cm) Length (cm)
19 24 5403190 · 15.5F Pristine™ Long-Term · 15.5-17.5F Dualator™ Dilator
Hemodialysis Catheter · Tunneler
23 28 5403230 · 16.5F AirGuard™ Valved Introducer · 2 End Caps
with Peel-Away Sheath/Dilator
· StruXure™ Guidewire, “J” Tip 0.035”1
28 33 5403280 · 8F Dilator
· 18G Introducer Needle
· 10-12F Dualator™ Dilator
33 38 5403330 · 2 Adhesive Dressings
· 14-16F Dualator™ Dilator
· ID Card
55 60 5403550 1
55 cm kit includes stainless “J” Tip 0.038"
Pristine™ Long-Term Hemodialysis Catheter
Indications: The Pristine™ Long-Term Hemodialysis Catheters are chlorhexidine) may be used to clean the catheter; however, care insertion. Avoid exit site at groin area. · DO NOT pull tunneler out
indicated for use in attaining short-term or long-term vascular should be taken to avoid prolonged or excessive contact with the of the primary incision at an angle. Keep tunneler straight to
access for hemodialysis, apheresis, and infusion. Access is attained solution. · The following antiseptics, chlorhexidine gluconate 4% prevent damage to the catheter tip. The catheter can be bent
via the internal jugular vein, subclavian vein, or femoral vein. (Hibiclens™), sodium hypochlorite (ExSept Plus™), povidone iodine slightly. · Care should be taken NOT to force the dilator sheath
Catheters longer than 40 cm are intended for femoral vein (Povidone ointment, Betadine™ solution) and hydrogen peroxide introducer assembly into the vessel during insertion as vessel
insertion. Catheters may be inserted percutaneously. can be used on the catheter and at the exit site; however, care damage including perforation could result. As reported in
Contraindications: · Thrombosed vessels · Confirmed infection, should be taken to avoid prolonged or excessive contact with the literature, left sided catheter placement may provide unique
bacteremia or septicemia · Inadequate anatomy for placement solution. Solution should be allowed to completely dry before challenges due to the right angles formed by the innominate vein
of the device · Known or suspected sensitivity to the device applying a dressing. Intermixing of these solutions has not been and at the left brachiocephalic junction with the SVC. · Cardiac
materials · Prior or unresolved venous thrombosis at the proposed tested and is not recommended. · The following antibiotics, arrhythmias may result if the guidewire is allowed to touch the
placement site. mupirocin 2% ointment, Polyspoirn™ ointment and gentamicin can walls of the right atrium. · Care should be taken not to advance the
be used on the catheter and at the exit site. · Avoid excessive split sheath too far into vessel as a potential kink would create an
Warnings and Precautions: · The catheter should be inserted tightening of catheter’s connections when connecting bloodlines,
and removed only by a qualified, licensed physician or other impasse to the catheter. · To prevent air embolism and/or blood
caps or syringes. Overtightening might crack the connections. · Do loss, place thumb over the exposed orifice of the sheath introducer.
healthcare professional authorized by and under the direction of not clamp the dual lumen portion of the catheter; clamp only the
such physician. · The medical techniques and procedures described · Ensure that the introducer sheath is only torn externally. Catheter
extensions. Use only smooth-jawed forceps for clamping when not may need to be further pushed into the vessel as sheath is torn. ·
in these instructions do not represent ALL medically acceptable using the clamp supplied with the catheter. · Clamping the
protocols, nor are they intended as a substitute for the physician’s For optimal product performance, do not insert any portion of the
catheter repeatedly in the same spot could weaken the tubing: cuff into the vein. · Do not allow the catheter to move out of the
experience and judgment in treating any specific patient. · The change the position of the clamp regularly to prolong the life of
content of this pack is supplied EO (Ethylene Oxide) STERILE, non- vein with the sheath. Ensure that the vein is not bleeding around
the tubing. Avoid clamping near the adapter and hub. · Exercise the catheter. · To avoid damage to vessels and viscus, infusion
pyrogenic. · Use aseptic technique during catheter insertion, use, caution when using sharp instruments near the catheter. Catheter
maintenance and removal. · Do not use the device if the “Use-By” pressures should not exceed 25 psi (172 kPa). The use of a 10 mL or
tubing can tear when subjected to nicks, excessive force, or rough larger syringe is recommended because smaller syringes generate
date indicated on the package label has passed. Do not use the edges. · Inspect the catheter frequently for nicks, scrapes, cuts, etc.
catheter if package has been previously opened or damaged. more pressure than larger syringes. · Do not suture through any
which could impair its performance. · When injecting heparin part of the catheter. · Acetone and PEG-containing ointments can
Inspect the device package and content to verify that no damage solution, inject quickly and clamp extension while under positive
has occurred as a result of shipping, handling and/or storage. If cause failure of this device and should not be used with
pressure. Heparin solution volume to lock each lumen must be polyurethane catheters. Chlorhexidine patches or bacitracin zinc
damage to the sterile barrier or the device is noted, do not use the equal to the priming volume of each lumen. Priming volumes are
device. Retain the package with the contents and notify your BD ointments (e.g., Polysporin ointment) are the preferred alternative.
marked on each lumen. · Remove the catheter as soon as it is no · Before flushing, pull the plunger back to verify blood flow and to
representative. · Single patient use only. Do not reuse, reprocess or longer necessary. · Catheter removal should be performed by
re-sterilize. Reuse, reprocessing or re-sterilization may compromise ensure that there are no blood clots. Do not flush clots through the
adequately trained healthcare professional or delegate. During catheter (see Thrombi Formation). · Never forcibly flush an
the structural integrity of the device and/or lead to device failure, catheter removal, do not cut the catheter prior to removal from the
which may result in patient injury, illness or death. Reuse, obstructed lumen. · Thrombolytic agents may cause systemic
vein to prevent the occurrence of an air embolism. If there is fibrinolysis if infused into circulation. Refer to the manufacturer’s
reprocessing or re-sterilization may create a risk of contamination resistance as the catheter is being withdrawn from the vein, avoid
to the device and/or may cause patient infection or cross-infection, instructions, indications for use and contraindications before
aggressive pulling to reduce the resistance. · Free the cuff and using Thrombolytic agents. Stereptokinase is not recommended, it
including, but not limited to the transmission of infectious surfaces from the tissue prior to removal. When removing the
disease(s) from one patient to another. Contamination of the has been reported to be anaphylactogenic. · Keep the catheter
catheter, DO NOT use a sharp, jerking motion or undue force; this clamped at all times except for when connected to the bloodlines
device may lead to injury, illness or death of the patient. · Keep the may tear the catheter. · After use, dispose of the product and its
catheter extension tubing clamped at all times when not in use or syringe during treatment. · Alcohol should not be used to lock,
packaging in accordance with administrative and/or local, state soak or declot polyurethane dialysis catheters because alcohol is
and fill the catheter with sterile saline prior to implantation to and federal laws and regulations. · Never use after expiry date. · To
avoid air embolism. With each tubing change, purge air from the known to degrade polyurethane catheters over time with repeated
avoid damage to the vessels and viscus, infusion pressure must not and prolonged exposure. Hand cleaner solutions are not intended
tubing and aspirate any air from the catheter. · If the catheter is exceed 25psi (172 kPa); the use of a 10 mL or larger syringe is
intended to be placed in a internal jugular or subclavian vein, it is to be used for disinfecting BD hemodialysis catheter Luer-lock
recommended because smaller syringes generate more pressure connectors.
recommended to place the patient on a cardiac monitor during the than larger syringes. Subclavian access should only be used when
procedure for detection of arrhythmia. · To avoid vessel perforation no other upper-extremity or chest-wall options are available. · To Potential Complications: · Air embolism · Arterial puncture ·
and damage, do not forcibly insert the guidewire, dilators, or prevent air embolism, keep the catheter clamped at all times when Brachial plexus injury · Cardiac arrhythmia · Cardiac tamponade ·
valved pull-apart sheath/introducer. · Do not insert the valved pull- not attached to a syringe, IV tubing, or bloodlines. · Cannulation of Catheter erosion or extrusion through the skin · Catheter occlusion
apart sheath/introducer further than necessary: depending upon the left internal jugular vein was reportedly associated with a or breakage · Catheter thrombosis · Catheter tip migration or
patient size and access site, it may not be necessary to insert the higher incidence of complications compared to catheter placement malposition · Deep vein thrombosis - lower extremity · Endocarditis
entire length of the introducer into the vessel. · The valved pull- in the right internal jugular vein. · As reported in literature, left · Exit site infection · Exsanguination · Extravasation · Femoral
apart sheath/introducer is not a hemostasis valve. It is designed to sided catheter placement may provide unique challenges due to artery bleed · Femoral artery damage · Femoral artery dissection
reduce blood loss and the risk of air intake. · The valved pull-apart the right angles formed by the innominate vein and at the left · Femoral nerve damage · Femoral vein occlusion · Fibrin sheath
sheath/introducer is not intended to create a complete two-way brachiocephalic junction with the SVC. · If arterial blood is formation · Hematoma · Hemorrhage · Hemothorax · Hydrothorax ·
seal nor is it intended for arterial use. · When using a “J” end aspirated, remove the needle and apply immediate pressure to the Inferior vena cava injury · Intolerance reaction to implanted device
guidewire straighten the end allowing introduction into the site for at least 15 minutes. Ensure that the bleeding has stopped · Lower extremity ischemia · Mediastinal widening · Pneumothorax
introducer needle. Do not insert or withdraw the guidewire forcibly and that no hematoma has developed before attempting to · Pulmonary emboli · Pulmonary embolism · Retroperitoneal bleed
from any component: the wire could break or unravel. · After cannulate the vein again. · Do not pull back standard guidewire · Right arterial puncture · Sepsis · Subclavian artery puncture ·
placement of the catheter check for catheter tip location by over needle bevel as this could sever the end of the guidewire. The Subclavian vein stenosis · Subcutaneous tunnel infection · Superior
imaging. · Do not nick the catheter when suturing. · Do not introducer needle must be removed first. · If the microintroducer vena cava puncture · Thoracic duct injury · Thoracic duct laceration
excessively tighten the suture when tying at the venotomy site. · guidewire must be withdrawn while the needle is inserted, remove · Thrombosis of vein · Trauma to major vessel or right atrium ·
Prolonged exposure to ultraviolet light can damage the catheter. · both the needle and wire as a unit to prevent the needle from Tunnel infection · Venous stenosis
Acetone and Polyethylene Glycol (PEG)-containing ointments damaging or shearing the guidewire. · Place a thumb over the Please consult product labels and instructions for use for
should not be used with polyurethane catheters. · Alcohol orifice of the sheath to minimize blood loss and risk of air indications, contraindications, hazards, warnings and
disinfectants (or alcohol containing antiseptics, such as aspiration. · The risk of infection is increased with femoral vein precautions.
bd.com BD, Tempe, AZ, USA, 1 800 321 4254
BD, the BD Logo, AirGuard, Dualator, Pristine, StruXure, and Y-Tip are trademarks of Becton, Dickinson and Company or its affiliates. © 2021 BD.
All Rights Reserved. All other trademarks are the properties of their respective owners. Illustrations by Mike Austin. Copyright © 2021. BD-28602You can also read