The Oligomeric Stromal Proteome of Arabidopsis thaliana Chloroplasts* S
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Research
The Oligomeric Stromal Proteome of
Arabidopsis thaliana Chloroplasts*□ S
Jean-Benoit Peltier‡§, Yang Cai‡¶, Qi Sun储, Vladimir Zabrouskov‡**, Lisa Giacomelli‡,
Andrea Rudella‡, A. Jimmy Ytterberg‡, Heidi Rutschow‡, and Klaas J. van Wijk‡ ‡‡
This study presents an analysis of the stromal proteome in amino acids, vitamins, purine and pyrimidine nucleotides, tet-
its oligomeric state extracted from highly purified chloro- rapyrroles, and isoprenoids (1). Chloroplasts are required for
plasts of Arabidopsis thaliana. 241 proteins (88% with nitrogen and sulfur assimilation and contain numerous protein
predicted cTP), mostly assembled in oligomeric com- chaperones and assembly factors, peptidases, and proteases
plexes, were identified by mass spectrometry with em- (1). To facilitate chloroplast gene expression, chloroplasts
phasis on distinguishing between paralogues. This is crit- contain proteins associated with plastid DNA and the plastid
ical because different paralogues in a gene family often
transcriptional and translation machinery including many
have different subcellular localizations and/or different
mRNA-binding proteins involved in mRNA processing, stabil-
Downloaded from https://www.mcponline.org by guest on December 20, 2020
expression patterns and functions. The native protein
masses were determined for all identified proteins. Com- ity, and translation (2, 3). Predictions of the plastid proteome
parison with the few well characterized stromal com- by the subcellular localization predictors TargetP (4) and Pre-
plexes from A. thaliana confirmed the accuracy of the dotar (5) followed by correction with each reported sensitivity
native mass determination, and by extension, the useful- (0.85 and 0.82, respectively) and specificity (0.69 and 0.88,
ness of the native mass data for future in-depth protein respectively) suggested that all non-green plastid types and
interaction studies. Resolved protein interactions are dis- chloroplasts together contain between 1707 (for Predotar)
cussed and compared with an extensive collection of na- and 3454 (for TargetP) proteins (6).
tive mass data of orthologues in other plants and bacteria. In recent years, proteomic studies together with many de-
Relative protein expression levels were estimated from tailed “one-protein-at-a-time” studies are collectively begin-
spot intensities and also provided estimates of relative
ning to provide a good insight into the chloroplast proteome.
concentrations of individual proteins. No such quantifica-
The thylakoid and envelope proteomes of chloroplasts from
tion has been reported so far. Surprisingly proteins dedi-
cated to chloroplast protein synthesis, biogenesis, and fate Arabidopsis thaliana have been analyzed in a number of studies,
represented nearly 10% of the total stroma protein mass. which were reviewed recently (6 –9). No in-depth analysis of the
Oxidative pentose phosphate pathway, glycolysis, and Cal- highly purified stromal proteome of A. thaliana has been carried
vin cycle represented together about 75%, nitrogen assim- out to date but is urgently needed to complete the overview of
ilation represented 5–7%, and all other pathways such as the chloroplast proteome from A. thaliana rosette leaves. Man-
biosynthesis of e.g. fatty acids, amino acids, nucleotides, ual data mining of the A. thaliana chloroplast literature, such as
tetrapyrroles, and vitamins B1 and B2 each represented less is done at The Arabidopsis Information Resource (TAIR)1 (www.
than 1% of total protein mass. Several proteins with diverse arabidopsis.org/) and for the Plastid Proteome Database
functions outside primary carbon metabolism, such as the (PPDB; ppdb.tc.cornell.edu/) (10), will further help to provide an
isomerase ROC4, lipoxygenase 2 involved in jasmonic
acid biosynthesis, and a carbonic anhydrase (CA1), were
surprisingly abundant in the range of 0.75–1.5% of the 1
The abbreviations used are: TAIR, The Arabidopsis Information
total stromal mass. Native images with associated infor- Resource; PPDB, Plastid Proteome Database; OPPP, oxidative pen-
mation are available via the Plastid Proteome Database. tose phosphate pathway; ACCase, acetyl-CoA carboxylase; CN, col-
Molecular & Cellular Proteomics 5:114 –133, 2006. orless native; BN, blue native; LOX, lipoxygenase; EF, elongation
factor; GS2, glutamine synthase 2; SFBA, sedoheptulose/fructose-
1,6-biphosphate aldolase; TPI, triose-phosphate isomerase; ROC4,
rotamase CyP; CPN, chaperonin; CA, carbonic anhydrase; Rubisco,
Although best known for their role in photosynthesis, chlo- ribulose-1,5-bisphosphate carboxylase/oxygenase; RBCS, ribulose-
roplasts (and plastids in general) synthesize many essential 1,5-bisphosphate carboxylase/oxygenase small subunit; RBCL, ribu-
compounds such as plant hormones, fatty acids and lipids, lose-1,5-bisphosphate carboxylase/oxygenase large subunit;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RCA, ribu-
lose-1,5-bisphosphate carboxylase/oxygenase activase; Fd, ferre-
From the ‡Department of Plant Biology and the 储Computational doxin; Fd-GOGAT, ferredoxin-dependent glutamate synthase;
Biology Service Unit, Cornell Theory Center, Cornell University, Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; MDH,
Ithaca, New York 14853 malate dehydrogenase; NiR, nitrite reductase; ACP, acyl carrier pro-
Received, June 10, 2005, and in revised form, October 3, 2005 tein; DMRL, 6,7-dimethyl-8-ribityllumazine; PNPase, polynucleotide
Published, MCP Papers in Press, October 4, 2005, DOI 10.1074/ phosphorylase; RH, RNA helicase; TAP, tandem affinity purification;
mcp.M500180-MCP200 RAP, ribosome-associated protein; CTP, chloroplast transit peptide.
114 Molecular & Cellular Proteomics 5.1 © 2006 by The American Society for Biochemistry and Molecular Biology, Inc.
This paper is available on line at http://www.mcponline.orgThe Oligomeric Chloroplast Stroma
accurate overview of the chloroplast proteome. scale. As will be demonstrated here, we found that native gels,
Several chloroplast-localized biosynthetic pathways are such as colorless native (CN)-PAGE (or blue native (BN)-PAGE)
linked to each other with intermediates from one pathway followed by SDS-PAGE, currently provide the most convenient
being used in other pathways. In some cases, biosynthetic semiquantitative comparison of different protein species.
pathways branch into two different pathways such as heme In this study, we set out to (i) experimentally identify the
and chlorophyll biosynthesis at the level of protoporphyrin IX Arabidopsis stromal proteome with emphasis on distinguish-
(11). In other cases, several enzymes are shared by different ing between paralogues, (ii) determine the approximate and
pathways, such as enzymes in the Calvin cycle and oxidative relative accumulation levels of stromal proteins, (iii) determine
pentose phosphate pathway (OPPP) (12) or in the Calvin cycle the native masses of stromal proteins and, where possible,
and glycolysis (13). It has been demonstrated for several en- resolve protein interactions, (iv) collect information on plastid
zymes that specific protein isoforms or functional paralogues protein-protein interactions from A. thaliana or other plant
specialize in different functions or pathways often located in species, and (v) expand the PPDB as a plastid proteome
different subcellular localizations (e.g. cytosol versus chloro- resource for the plant community.
plast) or tissues (e.g. root versus shoot) (see e.g. Ref. 14). Thus
EXPERIMENTAL PROCEDURES
to understand the regulation of metabolic activity it is important
to distinguish between such functional paralogues. Tandem Plant Growth, Protein Preparations, and Protein Separation—
A. thaliana (Col 0) was grown under 10-h light/14-h dark cycles at
mass spectrometry with high mass accuracy will typically allow
Downloaded from https://www.mcponline.org by guest on December 20, 2020
25/17 °C. Rosette leaves were collected about 55 days after sowing.
distinguishing between such closely related paralogues. These plants were still in their vegetative stage, about 1 week prior to
Assembly and disassembly of multisubunit complexes as bolting. Intact chloroplasts were isolated, and the native stromal
well as suborganellar localization of enzymes (e.g. thylakoid proteome was collected, concentrated, and directly loaded on color-
membrane versus envelope membrane) has been shown to less native gels (CN-PAGE) as described in Ref. 16. The gels were
then loaded on Tricine-SDS-PAGE gels (linear gradients with 8 –15%
influence flux of different pathways. In some cases, this can
acrylamide), focused, and stained with Coomassie Brilliant Blue, silver
lead to so-called “metabolic channeling” (15). Protein-protein nitrate, or the fluorescent dye SYPRO Ruby (16).
interactions can also help to stabilize proteins and to protect Image Analysis and Quantification—Coomassie-stained gels were
against denaturation and proteolysis. These interactions can scanned with a desktop scanner or high resolution scanner (Amer-
be homomeric (between identical proteins) or heteromeric sham Biosciences), and SYPRO Ruby-stained gels were scanned
using a charge-coupled device camera (FluorS, Bio-Rad). Spot vol-
(between different proteins). Identifying these protein interac- umes were determined, corrected for background, and normalized to
tions is needed to truly understand plastid protein functions total spot volume using Phoretix software version 2004 (Non-linear,
and plastid metabolic pathways. Given the complexity of the Newcastle, UK). We always verified the correlation between predicted
stromal proteome, only a small number of stromal protein processed molecular mass and experimentally observed mass. In a
complexes in A. thaliana have been characterized. Examples limited number of cases, more than one protein was identified per gel
spot. We first verified whether the identities in a spot could be
are the heteromeric Clp protease complex of 325–350 kDa explained by any background signals (from streaking) from abundant
with 11 different proteins (16), the 200 –240-kDa heterotet- proteins in this gel area. Such identities from background were not
rameric ADP-glucose pyrophosphorylase (or glucose-1-phos- quantified. If it appeared that the identities of the proteins in a spot
phate adenylyltransferase) (17), the stromal signal recognition were truly the results of two co-migrating proteins and if the MOWSE
particle (18), and the ⬃150-kDa heterotetrameric tryptophan scores were in a similar range (within 3-fold difference), then each
protein was assigned half the spot volume. If spots contained more
synthase (19). Extensive searches of the published literature than one protein with very different MOWSE scores (at least a factor
(this study) did identify a significant number of plastid com- of 3), we removed the protein identification based on the lowest
plexes from a large variety of other plant species than Arabi- MOWSE score. This was a reasonable strategy because co-migrating
dopsis (spinach, pea, Brassica rapa, potato, barley, etc.), such proteins were typically of similar molecular mass. In case a protein
as the 30 and 50 S ribosomal subunits from spinach chloro- was identified more than once, we summed all corrected spot vol-
umes for that accession. To facilitate comparison of abundance of
plasts (20, 21), plastid pyruvate dehydrogenase (22), a plastid different proteins, the spot volume(s) for each accession was divided
hetero-oligomer of acetyl-CoA carboxylase (ACCase) of 600 – by the denatured molecular mass for each accession. This resulted in
800 kDa in soybean (23), and homo-octameric porphobilino- a rough approximation of relative concentration. We do point out that
gen synthase from pea plastids (24). Currently there is no protein abundance was calculated from SYPRO Ruby-stained spot
centralized data deposit of these protein-protein interactions intensity. Because SYPRO Ruby binds preferentially to charged res-
idues (lysine, arginine, and histidine) protein abundance is underesti-
in A. thaliana or other plant species. mated or overestimated if the proteins contain few or many charged
Another important aspect of understanding chloroplast func- residues, respectively.
tion is to determine protein expression levels and molar ratios Protein Identification by Mass Spectrometry, (Un)ambiguous Iden-
between different chloroplast proteins. Currently there is little tification, and Bioinformatics—Stained protein spots were excised,
knowledge of the relative accumulation levels of stromal pro- washed, and digested with modified trypsin, and peptides were ex-
tracted automatically (Progest, Genomic Solutions, Ann Arbor, MI).
teins even for the best studied chloroplast pathways. Quantifi- Proteins were identified by peptide mass fingerprinting using a
cation of molar ratios between proteins in complex proteomes is MALDI-TOF mass spectrometer (Voyager DE-STR, Applied Biosys-
generally difficult and has not been attempted at any large tems) and/or by tandem mass spectrometry using a capillary LC-ESI-
Molecular & Cellular Proteomics 5.1 115The Oligomeric Chloroplast Stroma
MS/MS (Q-TOF, Waters) as described in Ref. 25. MS or MS/MS information from the literature. The MapMan system has 35
spectra were used to search the predicted A. thaliana proteome main functional categories or Bins with a larger number of
downloaded from the TAIR database using an in-house installation of
Subbins (subcategories) (see also ppdb.tc.cornell.edu/map-
Mascot (www.matrixscience.com). Criteria for positive identification
from peptide mass fingerprinting and from MS/MS data are described man.aspx). To our surprise, proteins involved in folding, pro-
in Ref. 25. In the analysis of the MS data, an effort was made to teolysis, and sorting (Bins 29.3–29.9) represented the largest
distinguish between members of the same gene family or otherwise functional category in the stroma (14%) closely followed by
related gene products. In some cases, peptides were identified that proteins related to protein synthesis (Bins 29.1 and 29.2)
match to more than one protein. Uniquely matching peptides (diag-
(12%) (Table I). 21% of the identified proteins were involved in
nostic peptides) are then needed to determine which protein is ex-
pressed. In case the mass spectrometry data did match ambiguously secondary metabolism, covering amino acid metabolism
to more than one protein, these protein identifications are automati- (7%), nucleotide synthesis and degradation (4%), tetrapyrrole
cally linked within the PPDB database and reported in the tables. synthesis (6%), and enzymes involved in synthesis of vitamins
Plastid Proteome Database and New Interface—The construction B1 and B2, isoprenes, jasmonic acid, and lipids/fatty acids
of the PPDB (ppdb.tc.cornell.edu/) was originally described in Ref. 25.
(4%). As expected, proteins involved in primary carbon me-
The PPDB interface was improved, and search functions were ex-
panded since its inception in 2004. Also more detailed curated infor- tabolism, such as the Calvin cycle, OPPP, and glycolysis
mation can now be accessed. The annotated CN-PAGE gel image represented a population of significant number (12%) and
and associated experimental, predicted, and other data presented in abundance representing ⬃76% of the total stroma mass as
this study can be accessed via PPDB. PPDB also contains the the- determined from the relative spot intensities. Enzymes in-
Downloaded from https://www.mcponline.org by guest on December 20, 2020
oretical analysis of all Arabidopsis entries (currently release 5.0 of
volved in starch synthesis and degradation (Bin 2) were also
ATH1.pep with 29,161 nuclear encoded Arabidopsis proteins as well
as the mitochondrial and plastid genomes). Mascot scores, number of well represented. The function of 11% of the identified pro-
matching peptides, and highest peptide score for each identification teins was unknown (see the supplemental table and PPDB).
as well as functional classification are listed.
Miscellaneous—Chlorophyll concentrations were determined
The Identified Stromal Proteome Is Pure with High
spectrometrically in 80% acetone (26), and protein determinations
were done with the bicinchoninic acid assay (27). TargetP Prediction Rates
Cross-correlation of the identified proteins against other
RESULTS
plant proteomic studies on A. thaliana plasma membranes
Stromal Proteome Identification and Classification (30, 31), vacuole and tonoplast (32, 33), the peroxisome (34),
Purified intact chloroplasts were lysed under non-denatur- the nucleus (35), the cell wall (36), and the hydrophobic mito-
ing conditions, and chloroplast stromal proteins and protein chondrial membranes (37) and a dozen other mitochondrial
complexes were separated based on native mass using CN- proteome analyses (from www.mitoz.bcs.uwa.edu.au/; see
PAGE (28) followed by complete denaturation and separation Ref. 38) did not suggest obvious contaminants from non-
by SDS-PAGE. Proteins were visualized by Coomassie stain- chloroplast locations. Further analysis suggested two to four
ing of preparative gels for mass spectrometry analysis (Fig. 1, potential contaminants from the cytosol and mitochondria as
A and B) or by SYPRO Ruby staining of analytical gels for indicated in the supplemental table. In agreement with the low
quantification (Fig. 1, C and D). The smaller figures (Fig. 1, B number of contaminants, 88% of the identified nuclear en-
and D) are from independent preparations and show that coded proteins were predicted (TargetP, www.cbs.dtu.dk/
these CN-PAGE gel patterns are reproducible, supporting the services/TargetP/) to be plastid-localized. This is slightly
notion that CN-PAGE gels are an excellent tool for proteome higher than the reported 85% sensitivity (or true positive
analysis. Proteins were identified by MALDI-TOF MS peptide identification rate) for TargetP (4) and indicative of the high
mass fingerprinting and/or nano-LC-ESI-MS/MS followed by quality of this stromal data set. TargetP prediction for each
Mascot search against the TAIR database. In the analysis of protein is listed in the supplemental table. Seven proteins
the MS data, an effort was made to distinguish between were chloroplast-encoded.
members of the same gene family (paralogues). These search
results were automatically filtered using an in-house software Relation to Other Chloroplast Proteomic Studies
routine2 followed by manual verification and additional quality
About 40% (over 100 proteins) were not observed in earlier
control steps as detailed under “Experimental Procedures.”
A. thaliana chloroplast proteomic studies on the thylakoid and
241 non-redundant proteins were identified on the CN-
envelope membrane and their associated (stromal) proteins
PAGE gels (see the supplemental table and PPDB for inter-
(25, 39 – 46) (for cross-correlation, see the supplemental table
active searches). The identified proteins were classified ac-
and PPDB). Predicted functions of these newly identified pro-
cording to the hierarchical, non-redundant classification
teins include biosynthesis of amino acids, nucleotides, and
system developed for MapMan (29) (gabi.rzpd.de/projects/
proteins as well as secondary metabolism (e.g. tetrapyrrole,
MapMan/) adjusted after in-house manual verification and
thiamine, isoprenoids, and hormones), and numerous pro-
teins were without any predicted function.
2
Q. Sun and K. J. van Wijk, unpublished data. A significant number of proteins were listed in the chloro-
116 Molecular & Cellular Proteomics 5.1The Oligomeric Chloroplast Stroma
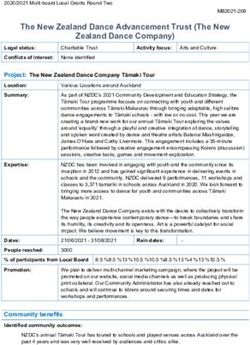
FIG. 1. Overview of gel-separated
proteins and protein complexes in the
chloroplast stroma of A. thaliana. The
stromal proteome was released by lysis
from isolated chloroplasts. Proteins
were separated by native gel electro-
phoresis (CN-PAGE) followed by dena-
turing SDS-PAGE and staining. The two-
dimensional electrophoresis gels were
either stained with Coomassie Brilliant
Downloaded from https://www.mcponline.org by guest on December 20, 2020
Blue (A and B) or with the fluorescent
dye SYPRO Ruby (C and D). The images
shown here are from several independ-
ent chloroplast preparations, demon-
strating the reproducibility of purification
and gel separation. Coomassie-stained
proteins spots were excised, digested
with trypsin, and identified by MALDI-
TOF MS and nano-LC-ESI-MS/MS. Gel
images and associated data (accession
numbers, native and denatured molecu-
lar masses, pI, spot volumes, and MS
data) can be accessed via the PPDB at
ppdb.tc.cornell.edu/. 241 proteins were
identified in the stroma. TKL, transketo-
lase; PGM, phosphoglucomutase; PGK,
phosphoglycerate kinase; SBPase, se-
doheptulose-1,7-bisphosphatase; PGP,
phosphoglycolate phosphatase; PRI,
D-ribose-5-phosphate isomerase.
plast analysis described in Ref. 47. However, this dataset is rently there is very little knowledge of the relative accumula-
problematic because it appears to contain a large percentage tion levels of the proteins in the chloroplast stroma. To this
(⬎40%) of non-chloroplast proteins as also reflected in the end, we quantified all 287 protein spots from analytical
low percentage of chloroplast predicted proteins. This is ei- SYPRO Ruby-stained CN-PAGE gels of two independent
ther a consequence of experimental contaminants and/or the chloroplast purifications and normalized those to total spot
result of high rates of “false identifications” during the search intensity (spot volume) of each gel. Proteins were identified in
with mass spectrometry data (for discussion see Ref. 6). 251 spots, representing ⬃99% of the total spot intensity (spot
volume). As is apparent from the gel images in Fig. 1, just a
Relative Abundance of the Identified Proteome handful of protein spots represent a large percentage of the
An important aspect of understanding plastid function is to total protein biomass of the stroma. The 23 most abundant
determine the expression level and molar ratios between dif- stromal proteins (based on normalized spot volume(s)) or
ferent plastid proteins in addition to their identification. Cur- based on “relative normalized concentration” (calculated from
Molecular & Cellular Proteomics 5.1 117The Oligomeric Chloroplast Stroma
TABLE I
Functional classification and abundance of the 241 identified proteins in the chloroplast stroma on the CN-PAGE gels
aa, amino acid; CHO, carbohydrates; OPPP, oxidative pentose phosphate pathway.
Number Number Relative amount
MapMan Binsa Functional classificationb
identified (percentage of total) of protein massc
%
1.1 Thylakoid 9 3.7 0.8
1.2, 1.3, 1.4, 1.7 Calvin cycle, OPPP, glycolysis 30 12.4 75.8
2, 3 Minor and major CHO 12 5.0 0.4
8 Organic transformation 6 2.5 1.4
12, 14 Nitrogen, sulfur assimilation 4 1.7 7.6
13 Amino acid synthesis and degradation 17 7.1 0.8
11, 16, 17, 18 Lipid, hormone, isoprene, vitamin, and cofactor 10 4.1 1.1
synthesis
19 Tetrapyrroles 14 5.8 0.5
20, 21 Redox and stress 20 8.3 1.9
23 Nucleotide synthesis and degradation 10 4.1 0.5
27 RNA 7 2.9 0.6
29.1 and 29.2 Protein synthesis 29 12.0 1.4
Downloaded from https://www.mcponline.org by guest on December 20, 2020
29.3–29.9 Protein targeting, folding, and degradation 34 14.1 5.2
24, 26, 28, 35 Unknown and miscellaneous 39 16.2 1.7
a
Functional assignment according to the MapMan Bin classification (29) and reported in PPDB.
b
Summarizing functional classification.
c
Relative protein amount for all proteins in this functional class normalized to the total amount on the CN-PAGE gel.
normalized spot volume divided by the experimental molec- 140 proteins of the 241 identified on our CN-PAGE gels,
ular mass of the protein) are listed in Table II. Together they together representing 82 different monomeric proteins or pro-
represent ⬃85% of the total stromal protein mass, and their tein complexes (supplemental table). For ⬃70% of those 140
expression covers a dynamic range of 2 orders of magnitude. identified proteins the native mass deduced from the CN-
These proteins were also prominent from independent “shot- PAGE gels is in approximate agreement with data from the
gun” nano-LC-ESI-MS/MS analysis of the stroma.3 We will literature. The native mass and estimated oligomeric state and
discuss these relative abundances further below, in connec- references for selected proteins are listed in Table III. The
tion with protein interactions and protein function. We do complete dataset is available in the supplemental table. Thus
point out, however, that protein abundance was calculated our comprehensive experimental native data provide a re-
from SYPRO Ruby-stained spot intensity. Because SYPRO source and starting point when searching for potential stromal
Ruby binds preferentially to charged residues (lysine, arginine, protein partners. One of the biggest surprises when searching
and histidine) protein abundance is underestimated or over- the literature was that about 60% of the proteins were iden-
estimated if the proteins contain few or many charged resi- tified as homo-oligomeric complexes or monomers rather
dues, respectively. than heteromeric complexes. The potential significance will
be discussed.
Native Mass, Oligomeric State, and Validation
As a first step in understanding the protein-protein interac- Integration of Native Masses, Relative Expression Levels,
tion network of the chloroplast stromal proteome, we deter- and Functions
mined the native mass of the identified proteins on the CN- In the remaining sections, we will highlight novelties and
PAGE gels (Fig. 1 and supplemental table). The gels were new insights concerning specific proteins and protein com-
calibrated with commercial native standards. The CN-PAGE plexes in terms of their relative abundance, native state, and
gels resolved proteins in a molecular mass range from ⬃ 20 to functions. To facilitate comparison of relative abundances of
⬃950 kDa with ribosomes and other large protein complexes different proteins, the spot volume(s) for each accession was
accumulating in the stacking gel in the first (native) dimension. divided by its denatured molecular mass, resulting in a relative
To compare these native mass data with existing data, we concentration. Given the inaccuracies and pitfalls of quantifi-
extensively searched the literature for mass information on cation from spot intensities, we simplified these relative con-
closely related and more distant orthologues in and outside of centrations to a scale of 1–5 with each level representing 1
plastids. Mass information from various species was found for order of magnitude (supplemental table). This provides an
immediate impression of the accumulation levels of the dif-
3
G. Friso, A. Rudella, H. Rutschow, and K. J. van Wijk, unpublished ferent functional categories as a group and for individual
data. proteins within a functional class.
118 Molecular & Cellular Proteomics 5.1The Oligomeric Chloroplast Stroma
TABLE II
The most abundant proteins in the chloroplast stroma and their functional classification, relative protein mass, and relative concentrations
The 23 most abundant proteins in terms of “relative protein mass” and in terms of relative concentration with their assigned functional
categories and predicted protein location are listed. These 23 proteins represent about 85% of the stromal protein mass. All protein spots on
the CN-PAGE gels of stroma were quantified based on spot “volume,” and volumes were normalized to the total spot volume. Proteins in the
spots were identified by mass spectrometry. Normalized spot volumes were converted in a measure of approximate relative concentration by
division of volume by the calculated mass (for details see “Experimental Procedures”). PS, photosynthesis; PP, pentose phosphate; TCA,
tricarboxylic acid cycle; org., organic; GAP, glyceraldehyde 3-phosphate dehydrogenase; Rib5P, ribulose-5-phosphate.
Relative
Accession Name MapMan Bin Ranka Volumeb
concentrationc
AtCg00490 RBCL 1.3.01 PS.Calvin cycle.Rubisco large subunit 1 (1) 46.02 0.852
At1g67090.1 RBCS-4 and other paralogues 1–3d 1.3.02 PS.Calvin cycle.small subunit 2 (2) 12.15 0.810
At5g35630.1 Glutamate-ammonia ligase (GS2), 12.2.02 nitrogen metabolism.ammonia 3 (3) 7.01 0.152
chloroplast metabolism.glutamine synthase
At3g60750.1 Transketolase (TKL) 1.3.07 PS.Calvin cycle.transketolase; 7.2 4 (6) 3.37 0.042
OPPP.non-reductive PP
At3g12780.1 Phosphoglycerate kinase-1 (PGK-1) 1.3.03 PS.Calvin cycle.phosphoglycerate kinase; 5 (9) 1.69 0.036
4.10 glycolysis.phosphoglycerate kinase
At3g62030.1 Peptidylprolyl isomerase ROC4 29.6 protein.folding 6 (4) 1.67 0.073
Downloaded from https://www.mcponline.org by guest on December 20, 2020
At2g21330.1 SFBA-1 1.3.06 PS.Calvin cycle.aldolase; 4.07 7 (8) 1.64 0.040
glycolysis.aldolase
At1g56190.1 Phosphoglycerate kinase-1 (PGK-2) 1.3.03 PS.Calvin cycle.phosphoglycerate kinase 8 (11) 1.39 0.029
At2g21170.1 TPI-1 (plastid) 1.3 PS.Calvin cycle 9 (5) 1.29 0.044
At3g01500.1 CA1 8.3 TCA/org. transformation.carbonic 10 (7) 1.05 0.041
anhydrases
At3g45140.1 Lipoxygenase AtLOX2, plastid 17.7.1 hormone 11 (22) 0.75 0.008
metabolism.jasmonate.synthesis-degradation
At5g20720.1 CPN21 29.6 protein.folding 12 (13) 0.65 0.027
At3g26650.1 Glyceraldehyde-3-phosphate 1.3.04 PS.Calvin cycle.GAP 13 (15) 0.65 0.017
dehydrogenase A-2 (GAPA-2)
At3g11630.1 2-Cys peroxiredoxin A 21.5 redox.periredoxins 14 (10) 0.62 0.030
At1g12900.1 Glyceraldehyde-3-phosphate 1.3.04 PS.Calvin cycle.GAP 15 (16) 0.62 0.016
dehydrogenase A-1 (GAPA-1)
At5g06290.1 2-Cys peroxiredoxin B 21.5 redox.periredoxins 16 (12) 0.61 0.028
At3g04790.1 Ribose-5-phosphate isomerase 1.3.09 PS.Calvin cycle.Rib5P isomerase; 7.2 17 (14) 0.58 0.021
(PRI) OPPP.non-reductive PP
At4g24280.1 cpHSP70–1 (DnaK homologue)e 29.6 protein.folding 18 (23) 0.57 0.007
At1g42970.1 Glyceraldehyde-3-phosphate 1.3.04 PS.Calvin cycle.GAP 19 (19) 0.56 0.013
dehydrogenase B (GAPB)
At3g55800.1 Sedoheptulose-bisphosphatase 1.3.08 PS.Calvin cycle.seduheptulose 20 (17) 0.55 0.014
(SBPase) bisphosphatase
At2g39730.1 Rubisco activase (R activase) 1.3.12 PS.Calvin cycle.Rubisco-interacting 21 (20) 0.52 0.011
At4g38970.1 SFBA-2 1.3.06 PS.Calvin cycle.aldolase; 4.07 22 (18) 0.51 0.014
glycolysis.aldolase
At4g20360.1 EF-Tu, chloroplast precursor 29.2.4 protein.synthesis.elongation 23 (21) 0.50 0.010
a
Rank determined by relative abundance measured in terms of relative spot volume; in parentheses, rank according to normalized
concentration.
b
Protein abundance as measured by spot volume with appropriate corrections.
c
Relative concentration with appropriate corrections.
d
In the case of RBCS with four genes (At5g38410, At5g38420, At5g38430, and At1g67090) and very high homology, mass spectrometry
measurements typically could not distinguish between the different paralogues.
e
cpHSP70-2 (At5g49910.1) appears to be expressed at slightly lower levels than cpHSP70-1 in the stroma.
Calvin Cycle, OPPP, Glycolysis, and Respiration (Bins 1.3, of the Calvin cycle mostly at levels 2 and 3, and specific
1.4, and 1.7)—We experimentally identified 27 proteins asso- enzymes of glycolysis and OPP at levels 3–5. We also iden-
ciated with the Calvin cycle, glycolysis, OPPP, and photore- tified 2-phosphoglycolate phosphatases 1 and 2 involved in
spiration, representing about 76% of the total stromal protein photorespiration at level 3 as a dimer. The Rubisco complex
mass (Table I). needs to be activated by the reversible carbamylation of a
The relative concentrations of these 27 proteins ranged lysine residue in RBLC (Lys-201) followed by rapid binding of
from level 1 to 5 with the small and large subunits of Rubisco magnesium. This process is regulated by Rubisco activase
(RBCS and RBCL, respectively) at level 1, the other enzymes (RCA) (48). The relative concentration of RCA (At1g73110)
Molecular & Cellular Proteomics 5.1 119TABLE III
Relative concentration and oligomeric state of selected proteins detected in the stromal proteome using CN-PAGE
120
References are listed in which such oligomeric state was determined in the published literature from A. thaliana, other plant species, or bacteria. Note that in the absence of published data on Arabidopsis
proteins this is information for homologues in closely related or more distant species. Additional parameters include the calculated mass of the monomeric protein and the native mass and relative concentration
as determined by CN-PAGE in this study. Functional assignment according to MapMan (29) is also listed. FA, fatty acid; CHO, carbohydrates; x, not relevant; HMW, high molecular weight; APS, ATP sulfurylase;
misc., miscellaneous; org., organic; cyt, cytosolic; TCA, tricarboxylic acid cycle.
Molecular mass Relative
Oligomeric normalized
Accessiona Laboratory annotationb Oligomeric statec state Organisme concentration MapMan Binj
referenced Precursorf Processedg Nativeh (⫻100)i
kDa
At5g46290.1 Ketoacyl-ACP synthase I Dimer 124 E. coli 50 64 129 0.00 11.1.03 lipid metabolism.FA synthesis and
(Lipid Gene Database; FA elongation.ketoacyl-ACP synthase
Beisson)
At1g29900.1 Carbamoylphosphate Heterodimer 63, 125 A. aeolicus, E. coli 130 123 174 0.19 13.1.2.3 amino acid
synthetase metabolism.synthesis.glutamate
family.arginine; 23.1.1 nucleotide
metabolism.synthesis.pyrimidine
At3g27740.1 Carbamoyl phosphate Heterodimer 63, 125 A. aeolicus, E. coli 47 101 163 1.24 13.1.2.3 amino acid
synthetase small subunit metabolism.synthesis.glutamate
The Oligomeric Chloroplast Stroma
family.arginine; 23.1.1 nucleotide
metabolism.synthesis.pyrimidine
At3g22890.1 ATP sulfurylase (ATPS1) Tetramer 62 Spinach 51 52 148 0.09 14.01 sulfur assimilation.APS
At2g02500.1 4-Diphosphocytidyl-2C- Likely a dimer This study A. thaliana 34 69 0.05 16.1 secondary metabolism.isoprenoids
Molecular & Cellular Proteomics 5.1
methyl-D-erythritol
synthase (ISPD),
mevalonate-independent
At3g45140.1 Lipoxygenase AtLOX2, Monomer This study A. thaliana 102 34 120 7.53 17.7.1 hormone
plastid metabolism.jasmonate.synthesis-
degradation
At2g44050.1 DMRL synthase (COS1) Homohexacontamer 68–70 E. coli and 24 17 738 3.59 18 cofactor and vitamin metabolism
(vitamin B2 synthesis) (60-mer) (icosaeder Bacillus, spinach
of 12 pentamers)
At1g03475.1 Coproporphyrinogen III Homodimer 71 A. thaliana 41 35 82 2.80 19 tetrapyrrole synthesis
oxidase
At1g48520.1 Glu-tRNA(Gln) Homodimer 72 C. reinhardtii 61 199 0.03 19 tetrapyrrole synthesis
amidotransferase subunit
B (GATB or GLU-ADT
subunit B)
At1g69740.1 Porphobilinogen synthase-1 Homo-octamer 24 Pea 47 41 343 0.16 19 tetrapyrrole synthesis
(␦-aminolevulinic acid
dehydratase-1) (ALAD-1)
At2g40490.1 Uroporphyrinogen Homodimer 126 Tobacco 44 40 126 0.30 19 tetrapyrrole synthesis
decarboxylase (UPD)
At3g25660.1 Glutamyl-tRNA Homodimer 72, 127 C. reinhardtii, 57 43 191 0.05 19 tetrapyrrole synthesis
amidotransferase subunit wheat
A
At5g24300.1 Starch synthase Homo- and 54, 55 Potato, maize 72 74 157 0.62 2.1.2.02 major CHO
heterodimer metabolism.synthesis.starch.starch
synthase
At4g09020.1 Glycoside hydrolase family Homotetra- to 128 Rice 86 67 96, 78, 84 0.04 2.1.2.04 major CHO
13, similar to isoamylase homohexamer metabolism.synthesis.starch.debranching
At3g29320.1 Starch phosphorylase-1 Homo- and 54, 55 Potato, maize 109 96 234 0.68 2.2.2.02 major CHO
heterodimer metabolism.degradation.starch.starch
phosphorylase
At1g03680.1 Thioredoxin m1 (Trxm1) Interacts with 129 A. thaliana 20 14 96, 89, 48 0.10 21.1 redox.thioredoxin
different targets
At1g50320.1 Thioredoxin X (TrxX) Interacts with 130 A. thaliana 20 13 154 0.04 21.1 redox.thioredoxin
different targets
At3g02730.1 Thioredoxin F1 (TrxF1) Interacts with 129 A. thaliana 19 13 155,212,170, 0.01 21.1 redox.thioredoxin
different targets 183
At2g20270.1 Glutaredoxin protein family Unknown None x 19 13 34 0.27 21.4 redox.glutaredoxins
At3g11630.1 2-Cys peroxiredoxin A Monomer, dimer, or 77, 78 Pea, barley 29 21 360,219,119, 29.59 21.5 redox.periredoxins
(PrxA) decamer 110, 58
Downloaded from https://www.mcponline.org by guest on December 20, 2020TABLE III—continued
Molecular mass Relative
Oligomeric normalized
Accessiona Laboratory annotationb Oligomeric statec state Organisme concentration MapMan Binj
referenced Precursorf Processedg Nativeh (⫻100)i
kDa
At3g52960.1 Peroxiredoxin II E (PrxE) Probably dimer This study A. thaliana 25 20 84 0.24 21.5 redox.periredoxins
At1g74260.1 Phosphoribosylformyl- Unknown None x 152 142, 39, 114, 0.04 23.1.2 nucleotide
glycinamidine synthase 93 metabolism.synthesis.purine
At2g35040.1 Phosphoribosylamino- Unknown None x 65 60 222, 213, 38, 0.09 23.1.2 nucleotide
imidazolecarboxamide 187 metabolism.synthesis.purine
formyltransferase
At5g48960.1 Similar to 5⬘-nucleotidase Unknown None x 73 67 166, 65 0.01 23.2 nucleotide metabolism.degradation
At5g63310.1 NDPK2, stromal Hexamer 81 Spinach 26 19 133 1.02 23.4 nucleotide
metabolism.phosphotransfer and
pyrophosphatases
At1g35420.1 Dienelactone hydrolase Monomer 96 Pseudomonas sp. 34 30 28 0.22 26.1 misc.misc2
family B13
At3g03710.1 3⬘–5⬘ exoribonuclease Homotrimer? 89, 90 Spinach 100 94 410 0.55 27.1 RNA.processing
At5g26742.1 DEAD box RNA helicase Unknown None x 81 45 980 0.03 27.1 RNA.processing
(RH3), mRNA etiolated
seedling
At1g77060.1 Isocitrate lyase and CoA Unknown None x 37 33 119, 147 0.01 27.3.99 RNA.regulation of
biosynthesis domain transcription.unclassified
At3g63140.1 Ribosome-associated Interacts with 70 S 84 C. reinhardtii 44 78 107, 126, 946, 0.58 27.4 RNA.RNA binding
protein (RAP41) ribosome 224
At1g75350.1 50 S ribosomal protein L31 Hetero-59-mer 20 Spinach 16 13 212 0.01 29.2.1 protein.synthesis.chloroplast-plastid
chloroplast ribosomal protein
At5g13650.1 Elongation factor protein; Unknown None x 74 29 980 0.32 29.2.4 protein.synthesis.elongation
typA/bipA like
At1g65260.1 Vipp1, mutant HCF155, Homohexacontamer 131 A. thaliana 36 30 932 0.68 29.3 protein.targeting
PspA-like (60-mer)?
At2g20890.1 THF1, thylakoid formation 1 Unknown None x 34 27 270 0.00 29.3 protein.targeting
At5g55220.1 Trigger factor Dimer 95 E. coli 62 60 146, 163 0.07 29.3 protein.targeting
At5g65620.1 Zinc oligopeptidase A (M3 Unknown None x 89 80 107 0.07 29.5.07
family) protein.degradation.metalloprotease
At1g36390.1 GrpE-2 Dimer/tetramer and in 93 C. reinhardtii 31 24 154, 197 1.95 29.6 protein.folding
complex with
HSP70
At2g28000.1 CPN60-␣-1 Heterotetradecamer 132 Narcissus 62 57 803 2.88 29.6 protein.folding
and HMW complex pseudonarcissus
with CPN21 when
ADP or ATP added
At2g44650.1 CPN10-1 Heptamer and 133 A. thaliana 15 11 170 1.50 29.6 protein.folding
multiple forms
At3g13470.1; CPN60--1,2,3 Heterotetradecamer 132 N. 63 46 807 2.24 29.6 protein.folding
At1g55490.1; and HMW complex pseudonarcissus
At5g56500.1 with CPN21 when
ADP or ATP added
At3g62030.1 Peptidylprolyl isomerase Unknown None x 28 36 107 72.78 29.6 protein.folding
ROC4
At4g24280.1 cpHSP70-1 (DnaK Heterotrimer 93 C. reinhardtii 77 68 123 7.26 29.6 protein.folding
homologue)
At5g17710.1 GrpE-1 Dimer/tetramer and in 93 C. reinhardtii 35 21 154, 202 1.27 29.6 protein.folding
complex with
HSP70
At5g20720.1 CPN21 (also CPN20) Multiple form and 133, 134 A. thaliana, 27 66 171 27.30 29.6 protein.folding
complexed with spinach
CPN60
At5g49910.1 cpHSP70-2 (DnaK Heterotrimer 93 C. reinhardtii 77 99 215 0.32 29.6 protein.folding
Molecular & Cellular Proteomics 5.1
homologue)
At5g50920.1 ClpC1 Dimer 16 A. thaliana 103 59 199 2.50 29.6 protein.folding
At5g66530.1 Aldose 1-epimerase Dimer 56 Aspergillus niger 34 30 61 0.31 3.5 minor CHO metabolism.others
The Oligomeric Chloroplast Stroma
121
Downloaded from https://www.mcponline.org by guest on December 20, 2020122
TABLE III—continued
Molecular mass Relative
Oligomeric normalized
Accessiona Laboratory annotationb Oligomeric statec state Organisme concentration MapMan Binj
referenced Precursorf Processedg Nativeh (⫻100)i
kDa
The Oligomeric Chloroplast Stroma
At1g11430.1 DAG protein-Related Unknown None x 26 20 929 0.06 33.99 development.unspecified
At1g21440.1 Carboxyvinyl- Unknown None x 36 32 119 0.00 35.1 not assigned.no ontology
carboxyphosphonate
phosphorylmutase
Molecular & Cellular Proteomics 5.1
At1g09340.1 Ribosome-associated Hetero-59-mer 84 C. reinhardtii 43 602, 151, 946, 0.69 35.2 not assigned.unknown
protein (RAP38) 604, 224,
107, 126,
890, 450,
319, 195,
230
At3g47520.1 Malate dehydrogenase Dimer 57 Sorghum 42 17 154, 323 1.31 8.2.09 TCA/org. transformation.other
(NAD), plastidic organic acid transformations.cyt MDH
At5g58330.1 Malate dehydrogenase Dimer 57 Sorghum 48 43 95, 109 3.52 8.2.09 TCA/org. transformation.other
(NADP) organic acid transformations.cyt MDH
a
Accession number from TAIR. Note that only gene model (typically .1) is indicated. However, in most cases the mass spectrometry data also matched the other gene models;
such details can be found in PPDB.
b
Internal annotation in PPDB.
c
Oliomeric state as reported in the literature.
d
Oliomeric state in A. thaliana or other species.
e
Organism used in the cited reference(s).
f
Calculated molecular mass of the precursor proteins as reported in PPDB.
g
Calculated molecular mass of the processed proteins as reported in PPDB.
h
Experimental native mass as determined from the CN-PAGE gels. If proteins were identified in more than one spot (e.g. due to aggregation, smearing, or truly different oligomeric
states), these additional native masses are also listed.
i
Relative concentration (multiplied by 100) in the stroma as determined by normalize spot volume divided by experimental mass. If more than one protein was identified in a spot,
the spot volume was divided by the number of its protein components.
j
Functional assignment according to the MapMan Bin classification (29) and reported in PPDB.
Downloaded from https://www.mcponline.org by guest on December 20, 2020The Oligomeric Chloroplast Stroma
was about 70 times less than RBCL and RBCS. Rubisco multiple native masses between ⬃100 and 360 kDa in agree-
N-methyltransferase catalyzing N-methylation of RBCL ment with observations of homo-, octa-, and decameric
(needed for an active enzyme) was ⬃14.000 times less abun- states (59, 60). A second paralogue, CA2 (At5g14740), was
dant than Rubisco. identified ambiguously with CA1 at low levels (level 5).
Many of the enzymes in these three pathways form hetero- Nitrogen and Sulfur Assimilation (Bins 12 and 14)—Plastids
or homo-oligomeric complexes. The 550-kDa Rubisco com- play a vital functional role in sulfur and nitrogen assimilation
plex is the most abundant and well studied example and with both elements used in amino acid biosynthesis. Chloro-
forms a hetero-oligomer with eight small and eight large sub- plasts import nitrite, which is converted by Fd-dependent
units. RCA was found in different native mass complexes nitrite reductase (NiR) into (toxic) ammonia followed by the
(330, 480, 600, and 770 kDa and as a ⬎950-kDa complex in glutamate- and ATP-dependent conversion into glutamine by
the stacking gel) and is known to transiently associate with the glutamine synthase 2 (GS2) and subsequent conversion into
Rubisco complex (for a review, see Ref. 49). Sedoheptulose/ glutamate by Fd- and ␣-ketoglutarate-dependent glutamate
fructose-bisphosphate aldolase 1 and 2 (SFBA-1 or -2) synthase (Glu1 or Fd-GOGAT-1). We identified and quantified
(At2g21330 and At4g38970) were both found at 178 kDa, each of these three key enzymes (At2g15620, At5g35630, and
suggesting a homo- or heterotetrameric state. These aldola- At5g04140) as a monomer (NiR) and at different native
ses are reported to form stromal homotetramers, whereas masses (GS2 and GOGAT). GS2 appears to have a high
aldolases outside the chloroplast form dimers (50, 51). relative concentration in particular as compared with NiR. This
Downloaded from https://www.mcponline.org by guest on December 20, 2020
Minor and Major Carbohydrate Metabolism (Bins 2 and is logical because 90% of the glutamine synthesized in leaf
3)—Starch and minor carbohydrate metabolism contribute to chloroplasts is derived from photorespiration rather than by
the carbohydrate storage and sugar diversity in plastids. We chloroplast import of nitrate and subsequent reduction to
identified 12 proteins in this functional category with relative nitrite (for discussion, see Ref. 61).
expression levels ranging from levels 3 to 5. These included ATP sulfurylase (ATPS1) catalyzes the formation of adeno-
two ADP-dependent pfkB carbohydrate kinases and SexI in- sine 5-phosphosulfate from inorganic sulfate and ATP. Four
volved in starch phosphorylation control (52, 53). Glucan paralogues (ATPS1– 4) are predicted to localize to non-green
phosphorylase-1 (At3g29320) (⬃110 kDa), involved in phos- and/or green plastids in A. thaliana. Cytosolic and chloroplast
phorolytic starch breakdown, forms a homodimer or het- isoforms were purified from spinach leaves, and their native
erodimer (54, 55). We identified it on the stroma native gels at mass was about 170 kDa as determined by gel filtration
233 kDa, corresponding to a dimer. ␣ isoamylase, a starch subsequent to other fractionation steps (62). We detected
debranching enzyme (At4g09020) of the hydrolytic or amylo- ATPS1 (At3g22890; ⬃49 kDa) at low levels (level 5) in the
lytic pathway, was found in the stroma as a monomer. Aldose stroma with a native mass between 129 and 147 kDa.
1-epimerase (At5g66530) catalyzes the interconversion of the Amino Acid Synthesis and Degradation (Bin 13)—We iden-
␣- and -anomers of hexose sugars such as glucose and tified 17 proteins involved in amino acid metabolism, repre-
galactose. Aldose 1-epimerase (35 kDa) acts as a dimer in senting about 0.8% of the stromal protein mass. They were
Aspergillus niger (56) and was indeed found on the CN-PAGE typically expressed at levels 3 and 4. Literature searches for
gel at 61 kDa. their oligomeric state suggest that most of these accumulate
Tricarboxylic Acid Cycle and Organic Transformation (Bin as dimers and trimers (between ⬃100 and 150 kDa) in differ-
8)—This functional class was represented by just five proteins ent plant species, and indeed these 17 proteins were found in
in agreement with the fact that these are mostly mitochondrial this mass range (supplemental table). As an example, we
functions (tricarboxylic acid cycle) or present in different sub- mention the carbamoylphosphate synthetase large
cellular compartments (carbonic anhydrases). We identified (At1g29900; 130 kDa) and small (At3g27740; 45 kDa) sub-
two types of malate dehydrogenases (MDHs), differing in the units. In Aquifex aeolicus and in Escherichia coli, they were
choice of cofactor (NADP or NAD) and activation mechanism. reported to form a heterodimer of 171 kDa (63). In appears
Chloroplast NADP-MDH (At5g58330) catalyzes the reduction that this oligomeric state is conserved in A. thaliana chloro-
of oxaloacetate into L-malate and is involved in the export of plasts because we found both subunits with a native mass
reducing power from the chloroplast to the cytosol (the malate between 162 and 173 kDa, corresponding to a heterodimer.
valve). We identified it predominantly at 323 kDa and to a Synthesis of Lipids (Bin 11), Hormones (Bin 16), Isoprenoids
lesser extent at 154 kDa. Chloroplast NADP-MDH (Bin 17), and Cofactors and Vitamins (Bin 18)—We identified
(At3g47520; 37 kDa) was suggested to form a homodimer six proteins involved in lipid/fatty acid biosynthesis, all of them
(57), and we identified it at 96 kDa. The accumulation level of at low levels (mostly level 5), clearly less abundant than pro-
the NADPH form was higher than the NAD form in agreement teins involved in e.g. amino acid biosynthesis. These include
with enzyme activity assays on purified Arabidopsis chloro- acetyl-CoA synthetase (acetate-CoA ligase) generating
plasts (58). The Arabidopsis genome encodes some 15 differ- acetyl-CoA from acetate (typically produced from glycolysis in
ent carbonic anhydrases. We identified an abundant (“at ex- mitochondria), ACCase, and one of the three ketoacyl-ACP
pression level 2”) carbonic anhydrase (CA1; At3g01500) at synthases, KAS1, as well as two desaturases, stearoyl-ACP
Molecular & Cellular Proteomics 5.1 123The Oligomeric Chloroplast Stroma
desaturases 1 and 2. KAS1 (At5g46290; 50 kDa) is an essen- oxidase (At103475; ⬃40 kDa) were reported to form a homo-
tial enzyme involved in the construction of unsaturated fatty octamer (24) and a homodimer (71). We identified each on the
acid carbon skeletons, and we identified it at 130 kDa. AC- CN-PAGE gel at ⬃340 and 82 kDa, which corresponds to
Case, catalyzing the first committed reaction of de novo fatty these reported oligomeric states. The A and B subunits of
acid biosynthesis, forms a heterotetrameric enzyme with plas- glutamyl-tRNA amidotransferase (At3g25660, 52 kDa; and
tid-coded subunit -carboxyltransferase, biotin carboxy car- At1g48520, 60 kDa) were identified at 190 –199 kDa on the
rier, biotin carboxylase, and ␣-carboxyltransferase. We did not CN-PAGE gel, strongly suggesting heterotetrameric interac-
identify these additional subunits most likely because they are tions. An ␣2 homodimer of 120 kDa has been identified in
primarily associated with the inner envelope membrane (64). Chlamydomonas reinhardtii (72).
We identified two proteins in plastid isoprenoid biosynthe- Stress Responses (Bin 20) and Redox Regulation (Bin 21)—
sis from the non-mevalonic acid or methyl-D-erythritol Many enzymes in plastids are activated and deactivated
4-phosphate pathway. These were 4-diphosphocytidyl-2C- through oxidation/reduction reactions via the thioredoxin sys-
methyl-D-erythritol synthase (ISPD or CMS) and GcpE/IspG/ tem. The thioredoxin family consists of nine proteins grouped
Clb4 immediately downstream in the pathway. Both accumu- in four clusters (m1,2,3,4; x; y1,2; and f1,2) (73–75). We iden-
lated at “level 4.” GcpE/IspG was only recently localized to tified five thioredoxins on the CN-PAGE gels; TrXm1 was
plastids (65). ISPD (At2g02500; ⬃30 kDa) was identified at 68 found at around 90 kDa, TrXm2 and TrXx were found at 154
kDa on the CN-PAGE gel, probably forming a dimer. kDa, and TrXf1 was found at both 154 and 212 kDa. Most
Downloaded from https://www.mcponline.org by guest on December 20, 2020
Plant hormones such as jasmonic acid, gibberellic acid, and likely they associate transiently with one or more different
abscisic acid and products of the terpenoid pathway are, in enzymes, but given the proteome complexity and the resolu-
part, synthesized in plastids. We identified lipoxygenase LOX2 tion of the native gels, we could not identify their respective
(At3g45140; 102 kDa) involved in jasmonic acid synthesis with partners. This is not surprising because recent affinity studies
a predominant native mass of 116 kDa, corresponding to a using modified thioredoxins as bait have shown that the thi-
monomer. It is surprisingly abundant, here quantified with a oredoxins interact with 50 or more chloroplast stromal pro-
relative concentration of level 3. We also identified it with high teins covering a wide range of functions (76).
Mascot scores in an earlier study as a thylakoid-associated We identified 14 proteins involved in oxidative stress re-
protein (43). It was shown that chloroplast-localized LOX2 is sponses. Some of them were quite abundant, such as perox-
required for the wound-induced synthesis of the plant growth iredoxins A and B at level 2. Peroxiredoxin IIE (At3g52960)
regulator jasmonic acid in leaves (66). was identified at a native mass of 84 kDa, whereas the abun-
Cofactors and vitamins are synthesized in plastids. We dant peroxiredoxins A/B were found at multiple native masses
identified two plastid enzymes involved in thiamine/vitamin B1 in agreement with reports from the literature (77–79). We also
biosynthesis, Thi1 (level 3) and ThiC (level 4), both accumu- identified several members of the ascorbate and glutathione
lating in complexes of 200 and 157 kDa, respectively (supple- defense systems, such as monodehydroascorbate reductase
mental table). To our knowledge, ThiC has not been identified and dehydroascorbate reductase-2, involved in recycling ox-
earlier in chloroplasts. Interestingly we found that Thi1 was idized ascorbate, and glutathione reductase. The native data
heavily oxidized as evidenced by high levels of methionine suggest that they might interact with each other in an 80 –
oxidation in the mass spectrometer. This high level of methi- 100-kDa complex, corresponding to a heterodimer.
onine oxidation was clearly specific for Thi1. It should be Nucleotide Synthesis and Degradation (Bin 23)—Plastids
noted that Thi1 is dually targeted to both plastids and mito- are a major site for pyrimidine and purine nucleotide synthe-
chondria, using two different translation initiation sites (67). sis, and indeed we identified 12 enzymes in these pathways
We also identified 6,7-dimethyl-8-ribityllumazine (DMRL) (all at levels 3 and 4), corresponding to about 0.5% of stromal
synthase (At2g44050) involved in vitamin B2 synthesis (ribo- protein mass. Two of them are shared with amino acid bio-
flavin). It was shown for the isoforms in E. coli and spinach synthesis. NDPK2 (At5g63310) is a nucleotide-diphosphate
that DMRL synthase is a 60-mer forming an icosaeder of 12 kinase involved in nucleotide metabolism (transfer of phos-
pentamers. In E. coli, each subunit is about 13–17 kDa, and phate from NTP to NDP). NPDK2 (⬃20 kDa) was reported to
the complex migrated around 850 kDa (68 –70). We found form a homohexamer (80, 81) in agreement with an observed
Arabidopsis lumazine synthase at 738 kDa, corresponding native mass of 133 kDa on the CN-PAGE gels. NDPK2 was
quite well with observations for the bacterial orthologue. predicted by TargetP and Predotar to be plastid-localized and
Tetrapyrrole Synthesis and Degradation (Bin 19)—We iden- was purified from spinach chloroplasts (81). Curiously NDPK2
tified nine proteins involved in tetrapyrrole synthesis (at “level is proposed to be a signal transducer in phytochrome-medi-
3”) and interestingly also the red chlorophyll catabolite reduc- ated light signaling, co-localizing with phytochrome in the
tase (also named “accelerated cell death 2” or ACD2) involved nucleus (80, 82). In light of the purification from spinach
in chlorophyll degradation (“level 4”). Together they totaled chloroplasts and its significant accumulation level in A. thali-
about 0.5% of the stromal protein mass. Porphobilinogen ana chloroplasts in this study, it seems less likely that NDPK2
synthase (At1g69740; ⬃45 kDa) and coproporphyrinogen III it is localized in the nucleus.
124 Molecular & Cellular Proteomics 5.1The Oligomeric Chloroplast Stroma
RNA (Bin 27) and Protein Synthesis and Protein Fate (Bin
29)—We were quite surprised to identify so many proteins (67)
assigned to Bins 27 and 29, together representing about 7%
of the stromal mass. The relative concentration among these
proteins spanned 3– 4 orders of magnitude with ROC4
(At3g62030; 20 kDa) as the most abundant protein. ROC4 is
an abundant stromal peptidylprolyl isomerase with demon-
strated in vitro rotamase activity, but its role is unclear (83).
ROC4 was found predominantly around 110 kDa and may
form a hexamer.
Spinach plastid 70 S ribosomes are composed of more
than 60 proteins and have a native mass of around 2 MDa (20,
21). The stromal 70 S ribosomes migrated just into the CN-
PAGE gel with low amounts of other large complexes (Fig. 1,
A and B). We analyzed 21 protein spots in this gel area, but
the analysis was not exhaustive. Nevertheless we identified
some 11 30 S subunits, 10 50 S subunits, and a plastid-
Downloaded from https://www.mcponline.org by guest on December 20, 2020
specific ribosome-associated protein (PSRP2). Ribosome-
associated proteins RAP41 (At3g63140) and RAP38
(At1g09340), originally identified in C. reinhardtii ribosomes
(84), were each found at three different locations of the stro-
mal CN-PAGE native gels: (i) in a complex larger than 950 kDa
most likely associated with 70 S ribosomes, (ii) at 224 kDa, FIG. 2. Examples of protein complexes and their match to pub-
and (iii) at 106 –126 kDa. At 224 kDa, the only obvious poten- lished literature. Relevant sections (native mass windows) of the
tial partners are ribosomal protein L5 (At4g01310) and ribo- CN-PAGE gels and the identified proteins in each mass window are
shown. The corresponding gel can be “interrogated” via the PPDB at
somal protein L31 (At1g75350) (Fig. 2A). At 106 –126 kDa, no ppdb.tc.cornell.edu. A, two plastid ribosome-associated proteins
obvious partners were found, suggesting the possibility that (RAP41, At3g63140; and RAP38; At1g09340) originally identified in C.
RAP38 and RAP41 form a heterotrimer. reinhardtii ribosomes (84) were both found at three different loca-
We identified elongation factors, EF-Tu-1 (At4g20360; 45 tions of the stromal CN-PAGE native gels. Here we show the two
kDa), ET-G (At1g62750; 78 kDa), and EF-P (At3g08740; 21 RAP proteins at ⬃224 kDa. Likely partners are ribosomal protein L5
(At4g01310) and ribosomal protein L31 (At1g75350). B, potential
kDa) as well a new elongation factor typA/bipA like protein interaction between cpHSP70-1/2 (At5g49910/At4g24280) and the
(At5g13650; 69 kDa). The BipA-like protein was found in a nucleotide exchange factors GrpE1/2 (At5g17710/At1g36390) ob-
complex over 950 kDa likely interacting with ribosomes. In served on the CN-PAGE gel from stroma.
E. coli, BipA is required specifically for the expression of the
transcriptional modulator Fis and binds to ribosomes at a site mal gel in a molecular mass complex of ⬎950 kDa. A plastid-
that coincides with that of elongation factor G (85). Elongation localized RH3 was identified in tobacco, and a loss of function
factor EF-Tu-1 found at multiple native masses ranging from mutant resulted in variegated leaves and abnormal roots and
110 to over 950 kDa was identified several times with high flowers (91). In E. coli, a DEAD-RNA helicase is part of the
Mascot scores (up to 732) and was abundant (level 2), sug- “degradosome” along with the PNPase, the endoribonuclease
gesting that it has additional functions. Indeed an orthologue RNase E, and the glycolytic enzyme enolase (92). RH3 in
in maize plastids was suggested to also serve as a chaperone chloroplasts does not seem to be associated with the PNPase
in particular during heat stress (86, 87). or At5g48960 mentioned above.
We identified several proteins involved in mRNA binding Chaperones cpHSP70-1 (At4g24280) and cpHSP70-2
and processing/degradation. Polyribonucleotide nucleotidyl- (At5g49910) are most likely in a complex of ⬃200 kDa with
transferase (At3g03710; ⬃95 kDa) acts as a 3⬘–5⬘ exoribo- GrpE1 (At5g17710) and GrpE2 (At1g36390) (Fig. 2B). In addi-
nuclease. We identified it in a stromal complex of 410 kDa. tion, GrpE1 and GrpE2 seem to form a complex at 150 kDa
This protein shows sequence homology to the polynucleotide without cpHSP70, but a potential pitfall is that transketolase is
phosphorylase (PNPase) of the E. coli degradosome. It pos- a major spot possibly masking cpHSP70. Indeed when ana-
sibly acts as a homotetramer (88 –90) and not as a hetero- lyzing the CN-PAGE gel of the peripheral thylakoid proteins,
oligomer as shown in other non-plant eukaryotes. We also where transketolase is less abundant, cpHSP70 was detected
identified At5g48960 (⬃65 kDa) encoding for a putative 5⬘- close to transketolase at ⬃150 kDa and most likely forms a
nucleotidase with unknown function; it was detected at 166 second type of complex with GrpE1–2 (not shown).
kDa in the stroma. At5g26742 encodes a putative DEAD box cpHSP70-1 and cpHSP70-2 (mostly the -1 isoform) also form
RNA helicase (RH3) and was identified on the CN-PAGE stro- complex at 123 kDa, but we did not detect any GrpE in this
Molecular & Cellular Proteomics 5.1 125You can also read