Case Report Metastatic Thyroid Cancer Presenting as Renal Cortical Mass
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Hindawi
Case Reports in Urology
Volume 2020, Article ID 9816479, 3 pages
https://doi.org/10.1155/2020/9816479
Case Report
Metastatic Thyroid Cancer Presenting as Renal Cortical Mass
Osamah Hasan ,1 Matthew Houlihan,2 Tobias S. Kohler,2 and Courtney M. P. Hollowell3
1
Midwestern University Chicago College of Osteopathic Medicine, Downers Grove, IL, USA
2
Department of Urology, Mayo Clinic, Rochester, MN, USA
3
Division of Urology, Cook County Health and Hospitals System, Chicago, IL, USA
Correspondence should be addressed to Osamah Hasan; ohasan15@midwestern.edu
Received 13 September 2019; Revised 11 November 2019; Accepted 4 December 2019; Published 6 January 2020
Academic Editor: David Duchene
Copyright © 2020 Osamah Hasan et al. This is an open access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The authors present a rare case of primary diagnosis of metastatic, differentiated thyroid cancer presenting as a solitary, large renal
mass. Renal cortical masses which represent metastatic primary malignancies are often small, multifocal, and in the setting of
active malignancy. Surgical excision of this patient’s renal mass demonstrated the unexpected diagnosis and subsequent endocrine
surgical intervention.
1. Introduction smoked and denied a family history of renal malignancy. On
physical examination, she was found to be in no acute distress,
The presentation, diagnosis, and treatment of renal cortical and appeared generally well (ECOG performance status 0).
masses, has evolved substantially over the last forty years. There were no carotid bruits or neck masses palpated. Her
Historically, the standard of care has been surgical excision chest was clear to auscultation bilaterally and her abdomen was
via partial or radical nephrectomy. Traditional therapy has soft, nontender, and nondistended without palpable abdominal
evolved in the modern era due in large part to increasing masses or costo-vertebral angle tenderness. Laboratory
incidence and earlier diagnosis of renal masses, which is due evaluation including comprehensive metabolic panel, complete
in large part to increased utilization of cross-sectional imaging blood count, and urinalysis were within normal limits.
in health care [1]. Management options of cT1 renal masses
may include active surveillance, thermal ablation, or 2.2. Imaging Studies. Computed tomography of the abdomen
extirpation via partial or radical nephrectomy depending on and pelvis with contrast demonstrated a 4.9 cm by 5.0 cm by
the size and location of the tumor, as well as patient-specific 5.8 cm endophytic interpolar left renal mass (Figures 1(a)
factors [2]. We report a unique urologic case with a primary and 1(b)). Chest CT with contrast demonstrated a 12 mm
diagnosis of differentiated thyroid cancer after undergoing right lower lobe pulmonary nodule, which was noted to have
nephrectomy for a cT1bN0M0 renal mass. been stable on prior imaging up to eleven years previously and
was interpreted as a granuloma. Finally, she was found to have
a slightly enlarged thyroid gland with several small calcified
2. Case Report nodules (Figure 2).
2.1. Patient Presentation. The patient was a 64-year-old female 2.3. Surgical Management. The patient was counseled
with a past medical history of hypertension, diabetes mellitus type regarding options for management and underwent hand-
2, and hyperlipidemia referred to urology clinic for evaluation assisted laparoscopic radical nephrectomy. The surgical
of a 5 cm left-sided renal mass that was incidentally discovered procedure was uncomplicated with an estimated blood
during evaluation for diarrhea and flank pain. She denied any loss of 25 cc. She tolerated surgery well, and, following an
history of gross hematuria or other urinary symptoms. She uncomplicated recovery, was discharged from the hospital
had no significant past medical history. The patient had never on postoperative day three.2 Case Reports in Urology
(a) (b)
Figure 1: CT A&P demonstrating a 4.9 cm × 5.0 cm × 5.8 cm renal mass. Coronal image (a), axial (b).
thyroid, which showed atypia. Subsequent nuclear medicine
whole body thyroid scan demonstrated no additional sites
of I-131 avidity. She underwent a total thyroidectomy and
radioactive iodine 3 months after. Pathology was consistent
with a follicular variant of papillary thyroid carcinoma.
3. Discussion
The differential diagnosis for pathologic etiology of cT1bN0M0
renal masses is broad. Renal cortical masses may be either
benign or malignant. Malignant renal cortical masses include
Figure 2: CT chest w/contrast demonstrating multinodular goiter. renal cell carcinoma, metastases from other malignancies and
renal medullary carcinoma [3]. Renal cell carcinoma is the
most common malignant cause of renal cortical masses and
can be divided into histologic subtypes including clear cell,
multilocular cystic clear cell, chromophobe, papillary,
collecting duct, unclassified, postneuroblastoma, and
mucinous tubular and spindle cell carcinoma [4]. Clear cell
renal cell carcinoma is the most common histologic subtype
representing roughly 75% of malignant renal masses with
papillary renal cell carcinoma representing 10–15% [4].
Patients with clear cell typically have a worse prognosis
compared with other histologic subtypes [5].
Differentiated thyroid cancer consists of both papillary
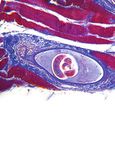
Figure 3: Photomicrograph showing follicular variant of papillary and follicular carcinomas. Differentiated thyroid cancer
thyroid carcinoma (H&E stain, 100x). typically remains localized to the thyroid and presents with a
thyroid mass. Incidence of distant metastatic disease has been
studied to be less than 4% [6]. The most common sites of
2.4. Patient Outcome. Histopathology revealed a 6.5 cm metastatic thyroid cancer are the cervical lymph nodes, lungs,
mass consistent with metastatic follicular variant of papillary bones, and brain [7]. It may also present with regional lymph
thyroid cancer (Figure 3). The diagnosis was confirmed with node metastasis [8]. There are rare case reports describing
immunohistochemistry as the tumor cells were CK7 (+), metastatic thyroid cancer involving the genitourinary organs
TTF1 (+), and Thyroglobulin (+) but CK20 (−) and WT1 (−). which include adrenal glands, kidneys, and penile and scrotal
The soft tissue and vascular surgical margins were negative skin [9, 10]. Thyroid cancer is an indolent diagnosis, with
for tumor. Given the results of the pathologic analysis and overall survival greater than 85% at 20 years when localized
evidence of several calcified nodules within the enlarged to the thyroid gland [11]. The prognosis of well-differentiated
thyroid, she was referred to endocrine surgery for further thyroid cancer is significantly worsened by distant metastatic
evaluation. On thyroid ultrasound, a left-sided thyroid nodule disease, with a 47-fold increase in risk of death [11]. Upon
was appreciated. Her endocrine evaluation included thyroid review of literature, 25 cases have been described involving
function panel, which demonstrated an elevated thyroid- metastatic differentiated thyroid cancer to the kidney [12].
stimulating hormone (TSH) of 7.57 uIU/mL and normal T4 Most cases described are in females over the age of 45
(0.81 ng/dL). She underwent fine-needle-aspiration of the years [12]. However, diagnosis of metastatic differentiatedCase Reports in Urology 3
thyroid cancer presenting as a primary renal mass has only American Journal of Surgical Pathology, vol. 27, no. 5, pp. 612–
been described in only two prior cases, per the authors’ review 624, 2003.
[13, 14]. Both patients had no other evidence of metastatic [6] A. R. Shaha, J. P. Shah, and T. R. Loree, “Differentiated thyroid
disease and as such underwent uncomplicated nephrectomies cancer presenting initially with distant metastasis,” The
with subsequent diagnosis of primary differentiated thyroid American Journal of Surgery, vol. 174, no. 5, pp. 474–476, 1997.
carcinoma [13, 14]. Both patients were treated post- [7] E. Farina, F. Monari, G. Tallini et al., “Unusual thyroid
nephrectomy with total thyroidectomies, with one patient carcinoma metastases: a case series and literature review,”
receiving post-thyroidectomy radioactive iodine. Follow-up Endocrine Pathology, vol. 27, no. 1, pp. 55–64, 2016.
data was available for one patient demonstrating freedom from [8] S. V. Jagtap, D. Patil, and C. S. Gupta, “Papillary carcinoma
local or distant metastases three years following radical thyroid presented with extensive local lymph nodal metastasis,”
nephrectomy [13]. Nonrenal cell carcinoma cortical metastases IP Archives of Cytology and Histopathology Research, vol. 3,
from other primary tumors are typically multifocal and no. 2, pp. 113–115, 2018.
bilateral, discovered in the setting of other metastases, and [9] M. O. Grimm, P. Spiegelhalder, H. Heep, C. D. Gerharz, H. D.
present as small, endophytic masses [15]. Our case discussed Röher, and R. Ackermann, “Penile metastasis secondary to
follicular thyroid carcinoma,” Scandinavian Journal of Urology
represented a solitary lesion, 5.8 cm in greatest dimension,
and Nephrology, vol. 38, no. 3, pp. 253–255, 2004.
with primarily exophytic components without evidence of
other sites of metastatic disease. [10] W. Shon, S. B. Ferguson, and N. I. Comfere, “Metastatic Hürthle
cell carcinoma of the thyroid presenting as ulcerated scrotum
nodules,” The American Journal of Dermatopathology, vol. 32,
no. 4, pp. 392–394, 2010.
4. Conclusion
[11] L. J. DeGroot, E. L. Kaplan, M. McCormick, and F. H. Straus,
In the setting of an enlarged thyroid gland with calcified nod- “Natural history, treatment, and course of papillary thyroid
ules during staging imaging, metastatic thyroid cancer should carcinoma,” The Journal of Clinical Endocrinology & Metabolism,
be included in the differential diagnosis of a localized renal vol. 71, no. 2, pp. 414–424, 1990.
mass. In such cases, baseline laboratory evaluation may [12] H. J. Song, Y. L. Xue, Y. H. Xu, Z. L. Qiu, and Q. Y. Luo, “Rare
include thyroid function test and preoperative consultation metastases of differentiated thyroid carcinoma: pictorial review,”
Endocrine-Related Cancer, vol. 18, no. 5, pp. R165–R174, 2011.
with endocrine surgery should be considered. Surgical exci-
sion of pT1bN0M0 renal mass via radical or partial nephrec- [13] L. D. Graham and S. M. Roe, “Metastatic papillary thyroid
tomy in an otherwise healthy female remains the standard of carcinoma presenting as a primary renal neoplasm,” The
American Surgeon, vol. 61, no. 8, pp. 732–734, 1995.
care. A multidisciplinary approach to postoperative follow-up
and care is essential in the setting of rare and unusual cases [14] F. P. Ruggiero, E. E. Frauenhoffer, and B. C. Stack, “Papillary
thyroid cancer with an initial presentation of abdominal and
such as that presented here.
flank pain,” American Journal of Otolaryngology, vol. 26, no. 2,
pp. 142–145, 2005.
Conflicts of Interest [15] K. Abe, T. Hasegawa, S. Onodera, Y. Oishi, and M. Suzuki,
“Renal metastasis of thyroid carcinoma,” International Journal
The authors declare that they have no conflicts of interest. of Urology, vol. 9, no. 11, pp. 656–658, 2002.
References
[1] J. M. Hollingsworth, D. C. Miller, S. Daignault, and B. K.
Hollenbeck, “Rising incidence of small renal masses: a need to
reassess treatment effect,” JNCI: Journal of the National Cancer
Institute, vol. 98, no. 18, pp. 1331–1334, 2006.
[2] R. J. Motzer, E. Jonasch, N. Agarwal et al., “Kidney cancer,
version 3.2015,” Journal of the National Comprehensive Cancer
Network: JNCCN, vol. 13, no. 2, pp. 151–159, 2015.
[3] J. McKiernan, O. Yossepowitch, M. W. Kattan et al., “Partial
nephrectomy for renal cortical tumors: pathologic findings and
impact on outcome,” Urology, vol. 60, no. 6, pp. 1003–1009,
2002.
[4] J. J. Patard, E. Leray, N. Rioux-Leclercq et al., “Prognostic value
of histologic subtypes in renal cell carcinoma: a multicenter
experience,” Journal of Clinical Oncology, vol. 23, no. 12,
pp. 2763–2771, 2005.
[5] J. C. Cheville, C. M. Lohse, H. Zincke, A. L. Weaver, and
M. L. Blute, “Comparisons of outcome and prognostic features
among histologic subtypes of renal cell carcinoma,” TheMEDIATORS of
INFLAMMATION
The Scientific Gastroenterology Journal of
World Journal
Hindawi Publishing Corporation
Research and Practice
Hindawi
Hindawi
Diabetes Research
Hindawi
Disease Markers
Hindawi
www.hindawi.com Volume 2018
http://www.hindawi.com
www.hindawi.com Volume 2018
2013 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018
Journal of International Journal of
Immunology Research
Hindawi
Endocrinology
Hindawi
www.hindawi.com Volume 2018 www.hindawi.com Volume 2018
Submit your manuscripts at
www.hindawi.com
BioMed
PPAR Research
Hindawi
Research International
Hindawi
www.hindawi.com Volume 2018 www.hindawi.com Volume 2018
Journal of
Obesity
Evidence-Based
Journal of Stem Cells Complementary and Journal of
Ophthalmology
Hindawi
International
Hindawi
Alternative Medicine
Hindawi Hindawi
Oncology
Hindawi
www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2013
Parkinson’s
Disease
Computational and
Mathematical Methods
in Medicine
Behavioural
Neurology
AIDS
Research and Treatment
Oxidative Medicine and
Cellular Longevity
Hindawi Hindawi Hindawi Hindawi Hindawi
www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018 www.hindawi.com Volume 2018You can also read