2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases - Nature
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
ARTICLE
https://doi.org/10.1038/s41467-021-22278-x OPEN
2D vanadium carbide MXenzyme to alleviate ROS-
mediated inflammatory and neurodegenerative
diseases
Wei Feng 1,2, Xiuguo Han 3, Hui Hu4,5, Meiqi Chang2, Li Ding2, Huijing Xiang1, Yu Chen 1,2 ✉ &
Yuehua Li4 ✉
1234567890():,;
Reactive oxygen species (ROS) are generated and consumed in living organism for normal
metabolism. Paradoxically, the overproduction and/or mismanagement of ROS have been
involved in pathogenesis and progression of various human diseases. Here, we reported a
two-dimensional (2D) vanadium carbide (V2C) MXene nanoenzyme (MXenzyme) that can
mimic up to six naturally-occurring enzymes, including superoxide dismutase (SOD), catalase
(CAT), peroxidase (POD), glutathione peroxidase (GPx), thiol peroxidase (TPx) and halo-
peroxidase (HPO). Based on these enzyme-mimicking properties, the constructed 2D V2C
MXenzyme not only possesses high biocompatibility but also exhibits robust in vitro cyto-
protection against oxidative stress. Importantly, 2D V2C MXenzyme rebuilds the redox
homeostasis without perturbing the endogenous antioxidant status and relieves ROS-induced
damage with benign in vivo therapeutic effects, as demonstrated in both inflammation and
neurodegeneration animal models. These findings open an avenue to enable the use of
MXenzyme as a remedial nanoplatform to treat ROS-mediated inflammatory and neurode-
generative diseases.
1 School of Life Sciences, Shanghai University, Shanghai, P. R. China. 2 State Key Laboratory of High Performance Ceramics and Superfine Microstructures,
Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, P. R. China. 3 Department of Orthopedic Surgery, Xin Hua Hospital Affiliated to
Shanghai Jiao Tong University School of Medicine, Shanghai, P. R. China. 4 Institute of Diagnostic and Interventional Radiology, Shanghai Jiao Tong University
Affiliated Sixth People’s Hospital, Shanghai, P. R. China. 5 Medmaterial Research Center, Jiangsu University Affiliated People’s Hospital, Zhenjiang, China.
✉email: chenyuedu@shu.edu.cn; liyuehua312@163.com
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 1ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x
R
eactive oxygen species (ROS) are chemically reactive
molecules containing oxygen, including singlet oxygen
(1O2), superoxide anion radical (O2−•), hydroxyl radical
(•OH), and hydrogen peroxide (H2O2)1. At low concentrations,
ROS play an essential role in adjusting cell functions2. However,
mismanagement and/or overproduction of ROS would subject a
biosystem to cause oxidative stress. Excess ROS result in irre-
versible oxidative damage to the biomacromolecules (e.g., lipids,
nucleic acids, and proteins), induce a variety of deleterious cel-
lular responses (e.g., apoptosis and necrosis), and trigger a myriad
of pathologies (e.g., atherosclerosis, neurodegeneration, inflam-
mation, aging, hemochromatosis, and even cancer)3,4. Therefore,
to remit its detrimental effect, tight ROS regulation is crucial for
maintaining cellular homeostasis.
Under normal circumstance, the intracellular redox equilibrium
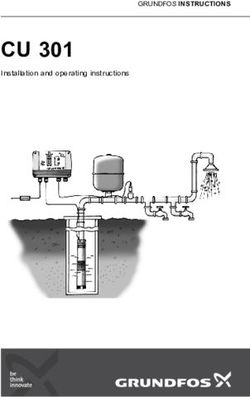
to resist oxidative stress is sustained by a collection of enzymatic Fig. 1 Schematic illustration of ROS-scavenging activities of V2C
antioxidants, primarily consisting of superoxide dismutase (SOD), MXenzyme with multiple enzyme-mimicking properties. V2C MXenzyme
catalase (CAT), peroxidase (POD), thiol peroxidase (TPx), glu- effectively catalyzes O2−• into H2O2 and O2, decomposes H2O2 into O2 and
tathione peroxide (GPx), etc.5–7. However, natural enzymes gen- H2O, and gets rid of •OH.
erally involve proteins and RNA molecules, which are susceptible to
environmental factors and become inactive under pathological activities, including SOD, CAT, POD, TPx, GPx, and haloperox-
conditions. To circumvent these drawbacks, artificial enzymes idase (HPO), which are in a position to catalyze ROS. Our results
termed as “nanozyme” with intrinsic enzyme-like characteristics demonstrate that 2D V2C MXenzyme effectively catalyzes O2−•
have been designed and constructed as alternatives to exert enzyme into H2O2 and O2, decomposes H2O2 into O2 and H2O and gets
functionality. Owing to their exceptional properties (facile pre- rid of •OH, as well as restrains ROS elevation by in vivo tests.
paration, high stability, tunable activity, etc.), nanozymes garner Intelligent cytoprotection against oxidative stress-induced inflam-
great attention and enable a broad spectrum of biomedical appli- mation and neurotoxicity can be achieved by the concerted cata-
cations. Recently, exciting paradigms can be found in the emerging lysis of multifunctional MXenzymes (Fig. 1, Supplementary Figs. 1
field of nanozymes, such as iron-based nanoparticles8–10, copper- and 2). This strategy not only sheds light on a type of nanozymes
based nanoreactors6, carbon-based nanoplatforms11,12, nanoceria13, with multiple enzyme-mimicking properties and excellent ROS-
noble metal-based nanomaterials (e.g., Au, Pt, and Pd)14–17, organic removal efficacy but also paves an avenue toward broadening the
nanoenzymes18–20, etc. Thereinto, vanadium, one of the 40 essential bioapplications of MXenes into catalytic nanomedicine.
micronutrients, possesses a regulatory role in the biological
system21,22. As a consequence of the intrinsic catalytic activities
toward classical peroxidase substrates and long-term anti-biofouling Results and discussion
abilities, vanadium-based nanomaterials have increasingly aroused Synthesis and characterization of 2D V2C MXene. 2D V2C
considerable interest in the field of nanozymes23,24. Despite the high MXene was synthesized via a facile exfoliation and intercalation
prospect, to the best of our knowledge, very few vanadium-based procedure (Fig. 2a)30. In a typical synthesis, the interlaced alu-
nanozymes have been developed so far24,25. These nanozymes, in minum layers were selectively extracted from the corresponding
particular, mainly concentrate on the single enzymic function MAX phase precursors V2AlC through wet-chemical etching
in vitro and almost neglect the in vivo effect. More importantly, using hydrofluoric acid (HF) solution. Then, the as-etched mul-
an artificial enzyme simply with a single function is incapable tilayered (ML) V2C MXene was delaminated by employing tet-
of mimicking the natural intracellular antioxidant system in com- rapropylammonium hydroxide (TPAOH) as an intercalant to
bating oxidative stress. The current trend in nanozyme develop- reduce the interaction between individual layers and subsequently
ment has focused on constructing a nanoreactor to simulate the obtain few-layered (FL) V2C MXene. As visualized by field-
sophisticated intracellular enzyme-participated ROS defense system. emission scanning electron microscopy (FESEM) images, pristine
Accordingly, it is necessary to develop more robust vanadium-based V2AlC exhibits closely compacted layered platelet morphology
nanomaterials that can functionally mimic the sophisticated cellular (Fig. 2b). After HF treatment, the compact layers expanded and
antioxidant enzymes. Recent advances in transition metal carbides became loose accordion-shape ML structure (Fig. 2c), demon-
and/or nitrides (MXenes) suggest that MXenes have been regarded strating the successful exfoliation. The energy dispersive X-ray
as a thriving class of two-dimensional (2D) materials in numerous (EDX) element mapping (Supplementary Fig. 3a), and corre-
potential applications ranging from energy storage to biomedicine, sponding elemental analysis (Supplementary Fig. 3b and Sup-
attributed to their versatile compositions, physicochemical diversity, plementary Table 1) confirm the uniform distribution of V, Al,
and tailorability26–29. Even though some impressive preliminary and C elements in MAX phase ceramic. In addition, a trimodal
results have been achieved in theranostics, biosensing, bioimaging, configuration of V, Al, and C in the hierarchical nanoflake
and antibacterial, the exploration of other scenarios of MXenes structure is further revealed by linear-scanning EDX spectra
in vitro and in vivo are still urgently required. along the lateral-section of the sample (Supplementary Fig. 4),
In this study, we report 2D vanadium carbide (V2C) MXene as a which shows that the signal areas of V and C are similar while the
successful paradigm of guiding nanozymes to implement anti- signal pattern of Al appears opposite to V and/or C.
oxidative behaviors for ROS elimination under pathophysiological TEM images and corresponding selected area electron diffrac-
conditions. The interdependent relationship between MXene and tion (SAED) pattern provide evidence that V2AlC is featured with
enzyme inspires us to propose the concept of “MXenzyme”: a laminar hexagonal crystal structure with a space group of P63/
MXenzyme is in analogy to the nomenclature of nanozyme, mmc (Fig. 3a–c). In order to investigate more details with the
highlighting enzyme-mimicking characteristics of the MXenes. We crystallographic structure and chemical composition, the atomic-
make the interesting discovery that 2D V2C MXenzyme is a kind resolution scanning transmission electron microscopy (STEM) in
of artificial nanozyme conducting intrinsic multiple enzyme-like high-angle annular dark-field (HAADF) mode was performed,
2 NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunicationsNATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x ARTICLE
a
Vanadium Carbon Aluminum
V2AlC MAX Phase Etching Al Atoms in HF Multilayer V2CTx Few-layer V2CTX
b c
1 μm 1 μm
10 μm 10 μm
Fig. 2 Synthesis and characterization of V2AlC MAX and V2C MXene. a Schematic diagram of selective etching and interlayer expansion route to
fabricate V2C MXene from the parent V2AlC MAX phase. b FESEM images (Inset depicts the corresponding high-magnification SEM image) and structural
model of V2AlC before HF treatment, displaying a typical compact layer structure. c FESEM images (Inset depicts the corresponding high-magnification
SEM image) and structural model of V2AlC after HF treatment to fabricate multilayer V2C MXene with a typical exfoliated layer topology. A representative
image of three replicates from each group is shown.
a b c i j k
5 nm-1 5 nm-1
200 nm 5 nm 200 nm 5 nm
e f m n
d l
5 nm-1
5 nm 1 nm 1 μm 100 nm
g h
6
0 4
o p
5 nm nm
I ntensity (×104)
V 4
3
Al
2
C 1 V Al C
1 nm Merge 0 1 2 3 4 200 nm 200 nm
Distance (nm)
q r s t u
42
nm
0
nm
2 μm 200 nm 1 μm 200 nm
Fig. 3 Structural and compositional characterizations of V2AlC MAX and V2C MXene. a Schematic of V2AlC structure. Top view of b low and c high
magnification (Inset describes the corresponding SAED pattern) TEM images of V2AlC. d Atomistic model of layer structure showing in-plane chemical
ordering in V2AlC MAX phase. e HRSTEM image (Inset illustrates the corresponding SAED pattern) and f enlarged HRSTEM images (upper presents
HAADF image and lower presents ABF image) of V2AlC. g HRSTEM with corresponding EDX mapping of V, Al, and C element signals of V2AlC along
[001] zone axis. h EDX linear-scanning profile of V, Al, and C element over the white line marked in the HAADF image. i Schematic of ML V2C MXene. Top
view of j low and k high magnification (inset exhibits the corresponding SAED pattern) TEM images of ML V2C MXene. l Atomistic model of layer structure
elucidating in-plane chemical ordering in ML V2C MXene. m, n Overview cross-sectional HRSTEM micrograph of ML V2C MXene after HF selective etching
of Al. o HAADF and p ABF image of ML V2C MXene. q Schematic of FL V2C MXene. r FESEM, s TEM, and t AFM image of FL V2C MXene after
intercalation with TPAOH. u TEM image of FL V2C MXene after sonication. A representative image of three replicates from each group is shown.
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 3ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x
which is sensitive to the element atomic number. It is worthwhile identifiable formazan at 450 nm6. It is noteworthy that the
to mention that the larger atomic number V (Z = 23) elements amount of formazan markedly decreased with increasing V2C
scatter the electrons more strongly than Al (Z = 13) elements, MXenzyme concentration (Fig. 4a), indicating that V2C MXen-
therefore, V atoms appear brighter contrast in this HAADF zyme is gifted with effective SOD-like activity.
images31. Cross-sectional HRSTEM images of V2AlC (Fig. 3e, f), CAT or CAT mimics have a well-documented capability to
as obtained along the [001] zone axis, signify a purity phase and catalyze the decomposition of two molecules of H2O2 for yielding
highly-ordered lamellar arrangement with alternating dark and O2 and H2O, therefore preventing the accumulation of H2O2
bright contrast, as schematically depicted in Fig. 3d. Furthermore, and protecting living organisms from oxidative damage by
the corresponding EDX elemental mapping of V, Al, and C atoms peroxide32. Terephthalic acid (TPA), a nonfluorescent probe,
in the sample reveals that the layer stacking order, excluding C, is reacts with H2O2 to be converted into a fluorescent indicator 2-
V–V–Al–V–V, where Al layer is sandwiched between two V2C hydroxyterephthalic acid with a fluorescence characteristic peak
layers (Fig. 3g). As illustrated in the EDX linear-scanning at 425 nm6. The fluorescent intensity displays a significant
analysis, the linear profiles imply a similar trend in variation of downtrend in the presence of V2C MXenzyme, demonstrating
V and C, whereas the dips for V and/or C are corresponding to that V2C MXenzyme can consume H2O2 (Fig. 4b, Supplementary
the peaks of Al and vice versa, undoubtedly proving that Al is Fig. 9). Subsequently, the CAT-like activity of the V2C
interleaved into two V2C (Fig. 3h). In addition, the distance MXenzyme was measured by monitoring the change in the
between two neighboring V layers is about 1.85 Å, the interval absorbance of H2O2 at 240 nm (Supplementary Fig. 10a)5. As
between V and Al is around 2.60 Å, and the separation between expected, there is a distinct reduction in the H2O2 absorbance as
two adjacent Al layers is approximately 6.65 Å. a function of time, suggesting that the V2C MXenzyme
After selectively etching the Al layers, the obtained ML V2C functionally mimics CAT. In addition, the CAT-like activity of
MXene presents the regular and homogenous layered structure V2C MXenzyme can also be testified by detecting the H2O2-
with hexagonal lattice features (Fig. 3i–k). It is noted that in the triggered O2 generation with the assistance of a dissolved-oxygen
cross-sectional overview micrograph (Fig. 3l–n), after treating meter. In the presence of V2C MXenzyme, O2 bubbles can be
with HF to remove Al from the densely packed MAX phase, the observed in the solution (Supplementary Fig. 10b) and the O2
cavities form, which is several to dozens of nanometers wide and production is increased with the concentration of H2O2
distributes periodically, indicates that the layers are split from (Supplementary Fig. 10c), providing the direct evidence that
each other. Careful inspection of atomic-resolution STEM images V2C MXenzyme could act as a kind of efficient CAT mimics to
(Fig. 3o, p), EDX element mapping (Supplementary Figs. 5a and scavenge H2O2. In order to identify the CAT-like enzymatic
6a) and corresponding elemental analysis (Supplementary catalysis mechanism, a steady-state kinetic assay was carried out
Figs. 5b, 6b, and Supplementary Table 2) prove that almost no by varying the concentration of H2O2 (2–400 mM) at a fixed
Al layer is remaining and the region is fully transformed into concentration of V2C MXenzyme, which followed the represen-
MXene. Notably, after TPAOH delamination, the exfoliated V2C tative Michaelis–Menten kinetics (Supplementary Fig. 10c–e).
MXene nanoflakes with a lateral size of several micrometers are POD is acknowledged as an another type of antioxidant
thin enough nearly to be electron-transparent (Fig. 3q–s), which enzyme that can detoxify the H2O2 to H2O33,34. In a standard
theoretically is a three-atom-thick material consisting of two procedure, 3,3′,5,5′-tetramethylbenzidine (TMB) was selected as
layers of V and one layer of C. As revealed in the atomic force the chromogenic substrate to assess the POD-like activity. In the
microscopy (AFM) measurements (Fig. 3t, Supplementary Fig. 7), presence of TMB, V2C MXenzyme catalyzes H2O2 into H2O.
the obtained MXene possesses a relatively uniform thickness of Meanwhile, the colorless TMB is converted into blue-colored
around 2.7 nm, a little thicker than the theoretical thickness, oxidized TMB (TMBox) with a maximum characteristic absor-
demonstrating that few layers of MXene were obtained, and the bance at 652 nm (Supplementary Fig. 11a, b), which is a
trapped water and other molecules could also contribute to the noteworthy time-dependent augmentation after the addition of
total thickness28. The STEM images with EDS mapping results the V2C MXenzyme into H2O2 aqueous solution (Fig. 4c), while
further confirm that Al element has been selectively etched, and the control groups without V2C MXenzyme or H2O2 display
V, C, F, and O atoms are uniformly distributed throughout the inconspicuous color variation (Supplementary Fig. 11a, b). V2C
entire nanoflakes, where F and O atoms originate from the MXenzyme possesses similar activities to natural POD, i.e., the
introduced groups of –F, –O, and –OH (Supplementary Fig. 8, POD-like activity of V2C MXene is pH sensitive (Supplementary
Supplementary Table 3). Finally, small and ultrathin 2D V2C Fig. 11c). In addition, after a time-scanning mode measurement,
MXene nanoflakes could be readily acquired by sonication the obtained curves followed the typical Michaelis–Menten
(Fig. 3u). equation confirming the POD-like behavior of V2C MXene
(Supplementary Fig. 11d–f).
GPx, an enzyme family with POD activity playing a critical role
Enzyme-mimicking activities of 2D V2C MXenzyme. The in maintaining H2O2 level, utilizes cellular tripeptide glutathione
multiple enzyme-mimicking properties of 2D V2C MXenzyme (GSH) as a reductant to catalyze the reduction of H2O2 to H2O
were assessed by selecting the ordinary substrates for natural accompanying by the transformation of reduced GSH to oxidized
enzymes under physiological conditions. Because O2−• is perpe- glutathione (GSSG), followed by reduction GSSG to produce GSH
tually generated in normal body metabolism, SOD, as a key with the assistance of GSH reductase (GR) and coenzyme
antioxidant enzyme in cells against ROS, catalyzes the dismuta- nicotinamide adenine dinucleotide phosphate (NADPH)5.
tion reaction of O2−• into O2 and H2O2, thus SOD mimetics can Accordingly, the GPx-like activity of V2C MXenzyme was
be employed as potential therapeutic agents against a number of determined by spectrophotometrically real-time measuring the
oxidative stress-triggered illnesses32. We initially explored the decrease of NADPH level at 340 nm (Fig. 4d)5,6. The absorbance
SOD-like activity of V2C MXenzyme. O2−• is commonly pro- of NADPH is reduced rapidly with time and up-regulated V2C
duced in situ through the reaction between xanthine (Xan) and MXenzyme concentrations (Fig. 4d, Supplementary Fig. 12a),
xanthine oxidase (XOD)33. The capability of V2C MXenzyme revealing the GPx-mimicking activity of V2C MXenzyme. The
to quench O2−• was investigated using 2-(4-iodophenyl)-3- GPx-like catalytic property of V2C MXenzyme was further
(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) (WST-1), estimated by a steady-state kinetic test employing H2O2 as the
which could work with O2−• to generate spectrophotometrically substrates (Supplementary Fig. 12b–d).
4 NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunicationsNATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x ARTICLE
a b c d
SOD-like activity CAT-like activity POD-like activity GPx-like activity
2O2
-
· + 2H+ → O +HO
2 2 2
2H2O2 → 2H O + O 2 2
H2O2 + TMB → TMBox + 2H O 2
H2O2 + 2GSH → GSSG + 2H O 2
30 0.49 0.8
I nhibitor rate of formazan
100 TPA Control V2C
Fluorescence (×106 a.u.)
0.42 0.7
Absorbance (a.u.)
TPA+H 2O2 V2C Control
24
Absorbance (a.u.)
80 TPA+H 2O2+V 2C
formation (% )
0.35 0.6
TPA+V 2C
60 18 0.28 0.5
40 12 0.21 0.4
20 0.14 0.3
6
0 0.07 0.2
0 0.00 0.1
0 200 400 600 350 400 450 500 550 600 0 20 40 60 80 100 0 50 100 150 200 250
Concentration (μg/mL) Wavelength (nm) Time (s) Time (s)
e H2O2 f
H2O2 GSH NADP+
y CAT
ctivit -like
like a
SOD- activ
ity
O2-· O2
GR
H2O2 H 2O
ivity
POD-like activity ke act
GPx-li e activity
k
TPx-li
H2 O GSSG NADPH
TMB
GSH/Cysteine
g h i
Trolox-equivalent antioxidant
1.6 150 120
scavenging activity (%)
Inhibition of ultraviolet
p = 0.0002
p = 0.0009
Hydroxyl radical
120 ***
capacity (mM)
1.4 90 p = 0.0002
radiation (%)
p = 0.0011 ***
***
p = 0.0009 **
90 p = 0.0003
***
1.2 p = 0.0094 60 ***
p = 0.0026
60 p = 0.034 ** **
* p = 2×10-6
1.0 30 ***
30
0.8 0 0
0.0 0.1 0.2 16 32 62.5 125 250 4 8 16 32 64
Concentration (mg/mL) Concentration (μg/mL) Concentration (μg/mL)
Fig. 4 Multiple enzyme-mimicking activities of 2D V2C MXenzyme. a SOD-like activity of V2C MXenzyme (n = 3 for each group). b CAT-like activity of
V2C MXenzyme (n = 3 for each group). c POD-like activity of V2C MXenzyme (n = 3 for each group). d GPx-like activity of V2C MXenzyme (n = 3 for each
group). e Schematic illustration of enzyme-mimicking activities of V2C MXenzyme. f Schematic representation exemplifying GPx-like activity of V2C
MXenzyme and GSH recycling by GR. g Total antioxidant capacity of V2C MXenzyme (n = 3 for each group). h Ultraviolet radiation-inhibition activity of
V2C MXenzyme (n = 3 for each group). i Hydroxyl radical-scavenging activity of V2C MXenzyme (n = 3 for each group). Data presented as mean ± SD,
and asterisks indicate a significant difference (*p < 0.05, **p < 0.01, and ***p < 0.001) as compared with the control group using one-way analysis of
variance (ANOVA).
Furthermore, assisted by other thiol-containing small molecules results provide compelling evidence that the as-constructed 2D
such as cysteine, V2C MXenzyme is endowed with an apparent V2C MXenzyme conducts six enzyme-mimicking activities under
TPx-mimicking behavior by catalyzing the reduction of H2O2 to the physiological condition, including SOD, CAT, POD, GPx,
water (Supplementary Fig. 13), indicating that V2C MXenzyme TPx, and HPO (Fig. 4e), which is superior as compared to CuxO
could utilize other thiol-molecules without GSH, which considers (three enzyme-like activities for CAT, GPx, and SOD)6, Prussian
the factual situation that the GSH homeostasis is commonly blue (three enzyme-like activities for CAT, POD, and SOD)33,
disturbed under oxidative stress35. It is worth noting that, in the Fe–N/C single-atom (four enzyme-like activities for POD, oxidase,
presence of halides (such as Br−), V2C MXenzyme also exhibits CAT, and GPx)36 and N-doped porous carbon nanospheres (four
evident HPO-like activity by catalyzing the reduction of H2O2 to enzyme-like activities for oxidase, POD, CAT, and SOD)11,
H2O and the oxidation Br− to form the corresponding hypohalous demonstrating that 2D V2C MXenzyme holds a broad-spectrum
acid (HOBr) (Supplementary Fig. 14). Taken together, these ROS removal capacity.
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 5ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x
The ROS-scavenging activity of 2D V2C MXenzyme. It is MXenzyme to the cell-culture medium effectively attenuates
essential to assess the ROS-scavenging activity of V2C MXenzyme H2O2-mediated oxidative damage and maintains cell viability,
and subsequently predict its potential for oxidation resistance. which is also V2C MXenzyme concentration-dependent (Fig. 5d,
Total antioxidant capacity (TAC), an important parameter for f). Fenton-like reaction, which produces highly toxic •OH from
assessing the antioxidant potentials, is usually expressed as Trolox the reaction between H2O2 and ferrous ion (Fe2+), has been
(TR) and Vitamin C equivalents. The results reveal that TAC broadly employed to induce cancer cell death (Fig. 5g, h)38.
value escalates with the elevated concentrations of V2C MXen- Distantly, V2C MXenzyme induces an apparent decrease in the
zyme (Fig. 4g, Supplementary Fig. 15). The ROS-scavenging production of ROS as caused by Fenton reagent, as well as
activity of V2C MXenzyme was further assessed by ultraviolet reduces Fenton regent-induced apoptosis, indicating the specific
(UV) protection activity since UV radiation has been used for capability to eliminate ROS. Confocal laser scanning microscopy
inducing ROS generation such as •OH and H2O237. Specifically, (CLSM) analysis further validates that V2C MXenzyme effectively
an apparent color fading is visually perceived for the reaction mitigates oxidative stress and thus protects cells from ROS-
system treated by V2C MXenzyme (Supplementary Fig. 16a), and induced cytotoxicity (Fig. 6).
the corresponding absorbance of the product oxTMB in the To ascertain the cofactor influence, the intracellular GSH and
reaction system with V2C MXenzyme is remarkably lower than GSSG levels were further monitored after different treatments
that in the absence of V2C MXenzyme (Supplementary Fig. 16b), (Fig. 5i, j). Buthionine sulfoximine (BSO), a compound that
which reveals the UV protection capacity of V2C MXenzyme. This blocks GSH biosynthesis25, reduces the cellular GSH levels.
activity is concentration-dependent, where a higher concentration Although no distinct differences in the GSH and GSSG levels
of V2C MXenzyme results in stronger UV protection efficacy were found after treatment with H2O2 or V2C MXenzyme alone,
(Fig. 4h). It is known that •OH is the most formidable kind of the addition of both H2O2 and V2C MXenzyme with BSO could
physiologically relevant ROS. Accordingly, the •OH-scavenging significantly diminish endogenous GSH level. Unlike the
activity of V2C MXenzyme was also examined, which demon- irreversible inhibition of BSO, the increased GSSG content did
strated that the signal intensity of •OH was notably decreased after not lead to much decreased GSH level in the presence of V2C
the treatment with V2C MXenzyme (Supplementary Fig. 17a–c). MXenzyme, which ascertains that V2C MXenzyme only employs
Nearly 70% of •OH was eliminated after exposure to 64 μg mL−1 GSH as a cofactor to get rid of H2O2, without disturbing the
V2C MXenzyme (Fig. 4i). Overall, the constructed 2D V2C transformation from GSSG to GSH. As a result, the efficient
MXenzyme possesses robust antioxidant enzyme-like activities intracellular uptake, noticeable ROS elimination, and impressive
and can efficiently scavenge miscellaneous ROS. cytoprotection of V2C MXenzyme enable it as an effective
artificial antioxidant enzyme to treat ROS-related diseases.
2D V2C MXenzyme for in vitro protecting cells against oxi-
dative stress. After polyvinyl alcohol (PVA) modification on the Protection of ROS-induced damage of intracellular compo-
surface, the 2D V2C MXenzyme displays high stability in various nents. The eventual outcome of ROS overproduction is oxidative
physiological solutions, including water, phosphate buffer solution stress-mediated apoptosis. As apoptotic effectors, a remarkable
(PBS), fetal bovine serum (FBS), and cell culture medium, with increment of caspase-3/7 activities was demonstrated to be
narrow size distribution and no obvious aggregation, guaranteeing involved in ROS-associated cytotoxicity in both L929 cells (Sup-
further biomedical applications (Supplementary Figs. 18–20). Cell plementary Fig. 25) and PC12 cells (Fig. 7a) by CLSM imaging. It is
counting kit-8 (CCK-8) assay was performed to assess the cyto- noted that the treatment with V2C MXenzyme results in a per-
compatibility of V2C MXenzyme. Different cell types such as ceptible fluorescence reduction. Subsequently, according to the
murine L929 fibroblasts and phaeochromocytoma cells (PC12) results of the Western blot assay, ROS-induced apoptosis as evi-
showed no visible cytotoxicity even the V2C MXenzyme was added denced by the expression of cleaved caspase-3 (Fig. 7b). Further-
at as high as 200 μg mL−1 (Supplementary Fig. 21). The cellular more, the levels of cleaved caspase-3 activity for Fenton’s reagent
uptake of both L929 and PC12 cells towards V2C MXenzyme was treatment are obviously higher than H2O2 or UV treatment, fur-
carried out to reveal their internalization behavior. After 24 h V2C ther confirming the high toxicity of •OH radicals. However, all the
MXenzyme exposure, the presence of V element in both cells was expressions of cleaved caspase-3 activity are immensely decreased
verified by EDX analysis (Supplementary Fig. 22a, b, e, f). TEM by the protection of V2C MXenzyme, which is ascribed to its ROS-
imaging also confirms the intracellular location of the V2C scavenging capacity, especially for •OH.
MXenzyme (Supplementary Fig. 22c, d, g, h). It is visible that V2C Intracellular ROS overproduction induces the oxidative damage
MXenzyme is mainly located at endocytic vesicles, suggesting of three kinds of bioactive macromolecules including proteins,
internalization via the endocytic pathway. It is found that either lipids, and DNA39,40. Subsequently, we explored the cytoprotec-
amiloride or nystatin has a significant inhibitory effect on endo- tive effect of V2C MXenzyme on intracellular protein carbonyla-
cytosis of RB-labeled V2C MXenzyme, revealing that the cellular tion, lipid peroxidation, and DNA damage. Protein carbonylation,
uptake of V2C MXenzyme in both L929 and PC 12 cells involves as the most common biomarker for ROS-induced irreversible
caveolae/lipid-mediated endocytosis and micropinocytosis (Sup- protein damage, is usually formed on the side amino acid chains
plementary Fig. 23). In addition, the amount of V2C MXenzyme in of lysine, proline, arginine, threonine, and tryptophan, which
both cells was quantified by inductively coupled plasma optical arouses conformational alternations in protein and directly leads
emission spectroscopy (ICP-OES, Supplementary Fig. 24). Both to the loss of protein function41. The administration of V2C
cells display a time-dependent accumulation of V element levels MXenzyme dramatically reduces the level of intracellular protein
when compared with the blank controls. carbonylation, indicating V2C MXenzyme plays a vital role in
Subsequently, we provided definitive identification that V2C defending protein against the accumulation of carbonylated
MXenzyme could act as an effective inhibitor of UV-modulated groups induced by ROS (Fig. 7c, d). Lipid peroxidation, an
ROS production and thus reduce the damage and cytotoxicity of irreversible process associated with cell or tissue necrosis, usually
UV irradiation on both L929 cells and PC12 cells (Fig. 5a, b). occurs late in the oxidative stress-induced injury42. The lipophilic
H2O2 induced obvious concentration-dependent cytotoxicity on fluorescent dye C11-BODIPY581/591 was used as a ratiometric
both L929 cells and PC12 cells since it could easily pass through probe for the determination of lipid oxidation, which readily
the cytomembrane (Fig. 5c, e). Notably, the addition of V2C enters the cells and undergoes fluorescence emission shifts from
6 NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunicationsNATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x ARTICLE
a 150
p = 1×10-7
150
c 120
d 150
p = 0.0003
250
g 150 150
*** ***
p = 3×10-4 p =6×10-5
200 p = 0.0006
Cell viability (%)
Cell viability (%)
Cell viability (%)
Cell viability (%)
90 *** p = 3×10-6
Intensity (a.u.)
Intensity (a.u.)
Intensity (a.u.)
p = 0.0009 *** ***
p = 0.0001 *** p = 0.0002
100 *** p = 2×10-6 100 100 ***
100 p = 0.0032 100
*** p = 4×10-9 p = 5×10-7
150 ** p = 1×10-5 ***
***
p = 0.0002 p = 4×10 -9
***
60 *** p = 2×10-9 *** -5
*** *** p = 1×10
p = 3×10-10 p = 0.1001 ***
100
50 50 *** 50 50 50
30 ***
*** p = 1×10-7 *** **
p = 1×10-5 50 p = 2×10-6
*** *** -12 p = 4×10-8 p = 0.0085
p = 9×10 *** ***
p = 2×10-13
p = 2×10-12 p = 0.9149 p = 3×10 -10 ** p = 5×10 -10 p = 0.7202
p = 0.0055
*** ***
0 0 0 0 0 0 0
Control 0 25 50 100 200 Control 100 200 400 Control 0 25 50 100 200 Control 0 25 50 100 200
Concentration (μg/mL) Concentration (mM) Concentration (μg/mL) Concentration (μg/mL)
L929 Cell viability L929 ROS intensity PC12 Cell viability PC12 ROS intensity
b 150 200 e f 150
p = 0.003
250 h 150
p = 3×10-8 ***
500
120 p = 0.002
p = 0.0009 p = 0.0137 **
** p = 0.001 200 p = 0.135 400
*** p = 0.4293
Cell viability (%)
*
Cell viability (%)
Cell viability (%)
Cell viability (%)
150 ** p = 0.1652
Intensity (a.u.)
Intensity (a.u.)
Intensity (a.u.)
p = 0.2539
100 p = 4×10-6 p = 8×10-7 90 p = 0.0009 100 100 p = 0.0761
*** *** 150 300
***
p = 1×10-10 100 p = 1×10-5 p = 0.0003
***
60 ***
*** 100 200
50 50 50
p = 7×10-7 p = 3×10-6 ***
p = 1×10-13
50 30 p = 4×10-5
p = 0.0102
p = 0.0037 *** *** ** 50 *** * 100
*** ***
*** ** p = 4×10-5 p = 0.0025 p = 0.0007 *
*** p = 2×10-12p = 0.1855 p = 8×10-7 p = 0.2308 p = 3×10-5 p = 0.0325
p = 4×10-13 ***
0 0 *** 0 0 0
0 0
Control 0 25 50 100 200 Control 100 200 400 Control 0 25 50 100 200 Control 0 25 50 100 200
Concentration (μg/mL) Concentration (mM) Concentration (μg/mL) Concentration (μg/mL)
i j
2.0 2.5
L929 PC12 L929 PC12
Fold GSSG level
Fold GSH level
p = 0.001
2.0 **
1.5 p = 0.0022
p = 2×10-5 **
p = 5×10-5 p = 0.0003 p = 2×10-5 p = 0.015
1.5 p = 0.0035
***
* **
1.0 *** p = 5×10-5 *** p = 7×10-7
*** p = 5×10-5
***
***
***
1.0
0.5
0.5
0.0 0.0
ol
ol
ol
2C
2C
O
O
O
O
ol
2C
2C
O
O
O
O
2
2
2
2
2
2
2
2
2O
2O
2O
2O
2O
2O
2O
2O
BS
BS
BS
BS
BS
BS
BS
BS
tr
tr
tr
tr
V
V
V
V
on
on
H
H
H
H
on
on
H
H
H
H
+
+
+
+
+
+
C
C
+
+
C
C
2
2
2
2
2C
2C
2C
2C
2O
2O
2O
2O
V
V
V
V
H
H
H
H
+
+
+
+
2C
2C
2C
2C
V
V
V
V
Fig. 5 Effect of V2C MXenzyme on intracellular ROS scavenging and cytoprotection. V2C MXenzyme protects L929 cells from oxidative stress as
induced by a UV irradiation (n = 6 for each group in cell viability and n = 4 for each group in ROS intensity), c, d H2O2 (n = 5 for each group in (c), n = 6 for
each group in (d) cell viability and n = 5 for each group in (d) ROS intensity), and g Fenton reagent (FeSO4 + H2O2, n = 4 for each group) via ROS
scavenging. V2C MXenzyme protects PC12 cells from oxidative stress as induced by b UV irradiation (n = 6 for each cell viability group and n = 4 for each
ROS intensity group), e, f H2O2 (n = 6 for each group in (e), n = 6 for each group in (d) cell viability and n = 4 for each group in (d) ROS intensity), and
h Fenton reagent (n = 4 for each group) via ROS scavenging. The central line represents the median (50th percentile), box limits represent the 25th and
75th percentiles and whiskers represent 1.5× the extent of the interquartile range. The cellular i GSH and j GSSG levels in both L929 cells and PC12 cells
after different treatments (n = 3 for each group). Data presented as mean ± SD, and asterisks indicated significant difference (*p < 0.05, **p < 0.01, and
***p < 0.001) as compared with the control group using one-way analysis of variance (ANOVA).
L929 cell PC12 cell
ROS L/D ROS L/D ROS L/D ROS L/D
Control H2O2 Control H2O2
200 μm 100 μm 400 μm 200 μm 100 μm 400 μm
V2C H2O2 + V2C V2C H2O2 + V2C
UV FeSO4 + H2O2 UV FeSO4 + H2O2
UV + V2C FeSO4 + H2O2 + V2C UV + V2C FeSO4 + H2O2 + V2C
Fig. 6 Effect of V2C MXenzyme on intracellular ROS scavenging and cytoprotection. CLSM images of L929 and PC12 cells with different treatments
stained with DCFH-DA or Calcein-AM/PI (L/D represents Live/Dead). A representative image of three replicates from each group is shown.
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 7ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x
a Control V2C UV UV + V2C H2O2 H2O2 + V2C H2O2 + FeSO4 H2O2 + FeSO4 + V2C
50 μm
50 μm
b L929 cell PC12 cell c d
carbonylation level
carbonylation level
250 L929 250 PC12
*** *
V2C - + - + - + - + - + - + - + - + p = 9×10‾5 p = 0.0139
Fold protein
Fold protein
200 *** 200
UV - - + + - - - - - - + + - - - - p = 0.0004
H2O2 - - - - + + + + - - - - + + + + 150 150
FeSO4 - - - - - - + + - - - - - - + + 100 100
Casp-3 35 kDa 50 50
0 0
Cleaved
17 kDa
2C
2C
2C
l
U UV
2C
2C
2C
l
+H 2
4
2+ 2
U UV
tr o
Fe Fe Fe 2
+H 2
4
2+ 2
tr o
Fe Fe Fe 2
2O 2O
O
2O
SO SO SO
2O 2O
O
2O
SO SO SO
Casp-3
V
V
V
V
V
V
V H2
on
V H2
on
H H
H H
V+
V+
4+ 4+
4+ 4+
C
C
2C
2C
GAPDH 37 kDa
Fig. 7 Protection of cellular components from ROS-induced damage by V2C MXenzyme. a CLSM images of Caspase-3/7 activity in PC12 cells after
different treatments. A representative image of three replicates from each group is shown in (a). b Western blot analysis of caspase-3, cleaved caspase-3,
and GAPDH expressions in both L929 cells and PC12 cells after different treatments. Protein carbonylation level of both c L929 cells and d PC12 cells after
different treatments (n = 4 for each group). Data presented as mean ± SD and asterisks indicated significant difference (*p < 0.05, **p < 0.01, and ***p <
0.001) as compared with the control group using one-way analysis of variance (ANOVA).
red to green when attacked by the product of lipid peroxidation43. 2′,7′-dichlorofluorescin-diacetate (DCFH-DA) was applied through
C11-BODIPY581/591-loaded cells with the stimulation of H2O2, in situ administration for in vivo detection of ROS correlated with
UV irradiation, and Fenton’s reagent demonstrate the anticipated inflammation. After PMA administration for six hours, the PBS-
red fluorescence decline and green fluorescence rise (Fig. 8a and treated ear unveiled strong fluorescence (Fig. 9b), indicating the
Supplementary Fig. 26). Nevertheless, the V2C MXenzyme-treated endogenous ROS generation. Comparatively, when V2C MXen-
groups display no distinct difference in the red fluorescence, zyme was injected into the site following PMA challenge, there was
triumphantly indicating that V2C MXenzyme can alleviate the a remarkable fluorescence decrease (a 56.5% reduction) in the
intracellular oxidative stress and inhibit the formation of lipid inflammatory ear (Fig. 9b, c), suggesting that V2C MXenzyme
peroxidation. possesses desirable ROS scavenging capability in vivo. Compared
ROS can induce several types of DNA damage, among which with hematoxylin and eosin (H&E)-stained images of the normal
DNA double-strand breaks (DSBs) can be potentially lethal to the mouse ear, inflammatory cell infiltration was visibly detected in the
cells44. Accordingly, we investigated the capability of the V2C PMA-treated ear (Fig. 9d), which evidenced PMA-induced
MXenzyme to protect DNA from ROS-induced DSBs. We activation of the inflammatory cascade. However, after treatment
analyzed the histone H2AX phosphorylated on Ser 139 with V2C MXenzyme, the symptom of cutaneous inflammation
(γ-H2AX) expression, which is a sensitive marker of DNA DSBs. was alleviated, demonstrating the high efficacy of V2C MXenzyme
Remarkably, an increment in γ-H2AX foci formation was against inflammation.
monitored in the V2C MXenzyme-untreated groups including To further enumerate and characterize the anti-inflammatory
H2O2, UV irradiation, and Fenton’s reagent, whereas an apparent effect of V2C MXenzyme, another acute ankle inflammation
decrease was observed in the cells pretreated with V2C model was stimulated by lipopolysaccharide (LPS) (Fig. 9e). After
MXenzyme, demonstrating that V2C MXenzyme confers a 5 h of LPS challenge, more than 1.3-fold growth in the ankle
protective effect on DNA integrity (Fig. 8b and Supplementary fluorescence intensity of LPS-treated mice was observed com-
Fig. 27). Moreover, highlighting the ROS-scavenging capability of pared with the untreated mouse (Fig. 9f, g), which was attributed
V2C MXenzyme, cleavage of calf thymus DNA is substantially to the enhanced ROS production by immune cells through the
suppressed by pretreatment with V2C MXenzyme in the presence activation of toll-like receptor 447,48. In contrast, the V2C
of Fenton’s reagent (Supplementary Fig. 28). MXenzyme-treated ankle showed enormously reduced fluores-
cence (a 27.3% reduction) (Fig. 9g), suggesting that V2C
In vivo anti-inflammation activity of V2C MXenzyme. The MXenzyme effectively suppresses LPS-induced inflammation.
in vivo toxicity of 2D V2C MXenzyme was initially evaluated. Pathological examination of ankle sections stained with H&E
The results of established hematology, serum biochemistry, and verified that more lymphocyte infiltrations were observed in the
histological assessment reveal that no appreciable inflammation, LPS-stimulated ankle whereas the treatment of V2C MXenzyme
hydropic degeneration, pulmonary fibrosis, hyperplasia, necrosis, remarkably attenuated the inflammation responses elicited by
and other abnormal phenomena were examined in the groups for LPS (Fig. 9h). These findings are in accordance with the anti-
4-weeks monitoring after intravenous injection of V2C MXen- inflammatory activity of V2C MXenzyme in PMA-induced ear
zyme at the dose of 15 mg kg−1 (Supplementary Figs. 29 and 30), inflammation.
collectively confirming that V2C MXenzyme is safe within our
tested dosage for further in vivo use. In vivo neuroprotection in MPTP-induced mice PD model.
Furthermore, acute local irritant dermatitis in the ear was Oxidative stress is associated with certain neurodegenerative dis-
initially established in mice by the topical application of phorbol eases, such as Parkinson’s disease (PD)49. Therefore, it encourages
12-myristate 13-acetate (PMA) (Fig. 9a), which induced a protein us to explore the therapeutic effects of V2C MXenzyme against
kinase C-mediated pronounced inflammatory response45,46. oxidative stress-mediated neurotoxicity. A PD mouse model was
8 NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunicationsNATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x ARTICLE
a Control V2C UV UV + V2C H2O2 H2O2 + V2C H2O2 + FeSO4 H2O2 + FeSO4 + V2C
50 μm
50 μm
20 μm
20 μm
b
50 μm
50 μm
Fig. 8 Protection of cellular components from ROS-induced damage by V2C MXenzyme. a CLSM image of lipid peroxidation of C11-BODIPY581/591-
stained L929 cells after different treatments. b Representative immunofluorescence CLSM images of γH2AX DNA damage foci in L929 cells after different
treatments. A representative image of three replicates from each group is shown.
initially established by using 1-methyl-4-phenyl-1,2,3,6-tetra- mice from neurotoxicity by inhibiting MPTP-induced oxidative
hydropyridine (MPTP), which is the gold-standard agent for stress.
replicating almost all of the PD symptoms50. MPTP, as a lipophilic
compound, can cross the blood–brain barrier, which was admi-
nistered intraperitoneally. Thereafter, V2C MXenzyme was Mechanism analysis of enzyme-mimicking activities. The typi-
implanted in solution into the striatum of MPTP-stimulated mice cal X-ray diffraction (XRD) pattern of V2AlC exhibits the pre-
(Fig. 9i, Supplementary Fig. 31). Tyrosine hydroxylase (TH), dominant peaks located at 13.47°, 35.55°, 36.24°, 41.27°, 45.28°,
the rate-limiting enzyme in dopamine biosynthesis, plays a vital 55.52°, 63.86°, 75.27°, and 78.86°, which is indexed with JCPDS
role in the pathogenesis and treatment of PD51. Therefore, after card No. 29-0101 (Fig. 10a). Compared with the MAX phase, a
different treatments, we further examined the alternations in the broad peak at around 6.05° corresponds to the (002) plane of
levels of striatal TH. Noteworthily, the TH levels were reduced MXene with a translation to the c lattice parameter of 14.5 Å,
remarkably in the MPTP-tread mice compared with those in the indicating the successful fabrication of V2C MXene53,54. Because
control group (Fig. 9j, k, Supplementary Figs. 32–34). In contrast, the surface chemistry is directly associated with the physico-
after V2C MXenzyme treatment, the mice displayed considerably chemical property, X-ray photoelectron spectroscopy (XPS), a
higher TH levels than only MPTP-stimulated mice, which indi- well-established technique to assess the chemical composition and
cated that V2C MXenzyme helps to maintain TH activity and valence states of V2C MXene, is performed to confirm the pre-
stability in parkinsonian mice. Compared with the untreated sence of V, C, O, and F elements (Supplementary Fig. 41a).
group, the upregulated expression of ionized calcium-binding Wherein, the V 2p region of V2C MXene, ranging from 510 to
adapter molecule 1 (IBA-1), a biological indicator of microglia 528 eV, is deconvoluted into four main peaks, which are assigned
activation, was distinctly observed in the striatum of the MPTP- to V–C (513.2 eV), V2+ (513.9 and 521.0 eV), V3+ (515.8 eV), and
treated group (Supplementary Figs. 35–37, Fig. 9l), reflecting that V4+ (523.0 eV) (Fig. 10b)55,56. The XPS C 1s spectrum is divided
the increased release of pro-inflammatory cytokines from micro- into five peaks of V–C, C–C, C–O, and O–C = O (Fig. 10c). The
glia could induce aggravated neuroinflammation52. On the con- O 1s are fitted with four contributions at 529.3, 530.0, 531.3, and
trary, V2C MXenzyme treatment effectively inhibites IBA-1 532.5 eV, which are corresponding to V–Ox, V–O, C = O, and
expression, suggesting that V2C MXenzyme could ameliorate the V–C–(OH), respectively (Fig. 10d)55. The F 1s peaks centered at
neuroinflammation of PD mice. Furthermore, it is commonly binding energies of 684.3 and 686.1 eV are assigned to V–F and
deemed that PD is ascribed to the striatal dopamine deficiency51. C–F bonds55 (Supplementary Fig. 41b).
4-Hydroxynonenal (4-HNE), a crucial bioactive marker of lipid For the mechanism of V2C MXenzyme as SOD mimetics, except
peroxidation, is a protein adduct of oxidative stress6. We then C element, we infer that V may function as the catalytic component
testified V2C MXenzyme for 4-HNE inhibition. As expected, in the superoxide catalysis by the reaction between VIV and VV
compared to the MPTP-induced PD group, there is a remarkable (Fig. 10e). Acting as an intermediate electron carrier, VIV can react
reduction in the expression of 4-HNE in the V2C MXenzyme- with superoxide and generate vanadyl hydroperoxide (VOOH),
treated group (Supplementary Figs. 38–40, Fig. 9m), further where an electron transfer from VIV to O2−•. Then, the protonation
indicating that V2C MXenzyme treatment significantly protects of VOOH accompanies by the H2O2 release and VV regeneration.
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 9ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x
a b Control V2C PMA PMA + V2C c 150 d
Fluorescence (% of PMA)
***
Max
Control
p = 0.0002
V2C
100
200 μm
50
PMA + V2C
PMA
0
ol
2C
2C
A
Min
tr
PM
V
V
on
+
C
A
e f g h
PM
150
Fluorescence (% of LPS)
LPS
Control
Control V2C LPS V2C + LPS LPS
***
V2C
p = 0.0007
100 200 μm
50
LPS + V2C
LPS
0
j
2C
ol
S
S
LP
LP
tr
Control MPTP MPTP + V2C
V
on
+
C
Immunohistochemistry
2C
V
i 1 mm
k l m 300
*** 400 *** ***
IBA-1 (% of control)
4-H N E (% of control)
150 p < 0.0001 p < 0.0001 p < 0.0001
TH (% of control)
300 200
100
50 μm
200
Immunofluorescence
100
50
100
1 mm 0 0 0
ol
ol
ol
P
P
P
P
P
P
PT
PT
PT
PT
tr
tr
PT
PT
tr
on
on
on
M
M
M
M
M
M
C
C
C
+
+
+
2C
2C
2C
V
V
V
50 μm
Fig. 9 Inflammation and neurodegeneration therapy based on V2C MXenzyme. a Scheme of ear inflammation model. b In vivo fluorescence imaging of
mice with different treatments to evaluate the effect of V2C MXenzyme on ROS scavenging in PMA-induced ear inflammation. c Corresponding radiant
efficiency of the fluorescence images acquired in the live mice after different treatments (n = 3 for each group). d H&E-stained images of mice ears after
different treatments. e Scheme of ankle inflammation model. f In vivo fluorescence imaging of mice with different treatments to evaluate the effect of V2C
MXenzyme on ROS scavenging in LPS-induced ankle inflammation. g Corresponding radiant efficiency of fluorescence images acquired in the live mice
after different treatments (n = 3 for each group). h H&E-stained images of mice ankles after different treatments. i Scheme of PD model treatment.
j Immunohistochemistry and immunofluorescence images of TH expression in the brains of mice after different treatments (coronal plane). Expression
levels of k TH, l IBA-1, and m 4-HNE in each treatment group (coronal plane) (n = 25 for each group), quantification represents the ratio of the
experimental group to control. Data presented as mean ± SD and asterisks indicate significant differences (***p < 0.001) using one-way analysis of variance
(ANOVA). A representative image of three replicates from each group is shown.
Thermodynamically, the dismutation of O2−• is concerned with the 1000 cm−1 corresponds to the bond of V-oxo (V = O) (Supple-
reduction and oxidation of O2−•, where the redox potential values of mentary Fig. 43)25. However, the vibration peak of V = O
E(O2/O2−•) and E(O2−•/H2O2) are 0.91 and −0.18 V, respectively57. disappears after adding H2O2 into the reaction system, indicating
As expected, the redox potential value of V2C MXenzyme reaches that V-peroxo species might be formed. Meanwhile, in the Raman
−0.11 V (Fig. 10f), which further confirms that the V2C MXenzyme spectra, the emerging of a weak band at 1200 cm−1 corresponds
is capable of catalyzing the O2−• dismutation. to V-peroxido species resulting from V2C MXenzyme treated
Based on the fact that vanadium possesses a different redox with H2O225, verifying that the V peroxide species 1 is generated
state by complexing with H2O2, the CAT-like activity mechanism on the surface of V2C MXenzyme. After further GSH treatment,
of the V2C MXenzyme is further revealed (Fig. 10g). When H2O2 the band of V = O at 1000 cm−1 in the FTIR spectrum and
is present in the reaction mixture, the VV species such as OVO+ 1200 cm−1 in the Raman spectrum does not reappear (Supple-
can be oxidized to form C1 monoperoxo vanadium species OV mentary Fig. 44), suggesting that the V = O reproduction for V2C
(O2)+58. Furthermore, the formed OV(O2)+ interaction with MXenzyme as GPx mimetics is not required, which proceeds
another H2O2 molecule results in the production of C2 diperoxo along with another pathway. According to the proposed
vanadium species HOOV(O)22+. The reaction between VV and mechanism (Fig. 10h), V2C MXenzyme provides reaction sites
H2O2 for the generation of VIV and •OOH was evidenced in the for H2O2 reduction accompanied by GSH oxidation. Subse-
electron spin resonance (ESR) spectrum measurement at liquid quently, GSH acting as a proton carrier protonates the partially
nitrogen temperature (−196 °C) (Supplementary Fig. 42). Fol- negatively charged oxygen (δ−), and GS− as the nucleophile
lowed by OV2+ supplement, the variable C3 m-peroxo bridge attacks the positively charged oxygen (δ+) in the meantime,
OVOOV(O2)+ breaks up to generate OVO+ and C4 oxo-peroxo generating a labile sulfonate-bound intermediate G2, which
radicals •OV(O2)2+, which is ascribed to the internal oxidation. disintegrates a G3 glutathione sulfenic acid (GSOH) and a G4
Finally, molecular oxygen (O2) is released from the reaction dihydroxo intermediate through the hydrolysis. It is noted that
system through the dismutation reaction of •OV(O2)2+. the GSOH produced by this pathway is likely the migration of
As evidenced in the Fourier transform infrared (FTIR) HOBr from an intermediate of V–OBr in vanadium-dependent
spectroscopy, the characteristic vibration of V2C MXenzyme at haloperoxidase24,25 (Supplementary Fig. 45). In the presence of
10 NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunicationsNATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-22278-x ARTICLE
a b c d
V 2p C 1s O 1s
V 2AlC Max Phase
C=C
Intensity (a.u.)
Intensity (a.u.)
Intensity (a.u.)
Intensity (a.u.)
V 3+
V 4+ V-Ox
V 2+ V-C O-C=O
C-O V-O
V-C C=O
V 2C MXene
V-C-(OH)x
JCPDS No.29-0101 103
116
002 101 104 106 110 200
004 100 109
10 20 30 40 50 60 70 80 528 524 520 516 512 290 288 286 284 282 280 535 534 533 532 531 530 529 528
2Theta (degree) Binding Energy (eV) Binding Energy (eV) Binding Energy (eV)
e g h
V V
V IV OVO+ O
H2O2
H2O2 V H 2O
V2C MXenzyme
+
(C1)
H+ H+ OV(O2) V2C MXenzyme
VOOH δ- O O δ+
H2O2 V
H2O2 -· H 2O GSH
O2
HOOV(O2)+ (C2) (G1) NADP+
SOD-like activity
OV2+
f GPx-like GR
H2O2 activity GS
OVIVOOV(O2)+ H+
2 HO O
(C3)
Current / mA/cm2
HO OH V
+
OVO V NADPH
1
· (C4)
OV(O2)+ (G2)
0 (G4) H2O GSSG
[VOV]
-1 (G3) GSOH
O2
CAT-like activity
-2 H2O
-0.6 -0.3 0.0 0.3 0.6 0.9 GSH
E / V vs. NHE
Fig. 10 Mechanism investigation of enzyme-mimicking activities. a XRD diffraction patterns of V2AlC MAX phase ceramic and V2C MXene. High-
resolution XPS spectra of b V 2p region, c C 1s region, and d O 1s region. e Schematic illustration on clarifying the underlying mechanism of SOD-like
activity of V2C MXenzyme. f Cyclic voltammogram of V2C MXenzyme showing their redox potential. g Schematic illustration revealing the related
mechanism of CAT-like activity of V2C MXenzyme. h Schematic illustration unveiling the mechanism of GPx-like activity of V2C MXenzyme.
enough GSH, the GSOH reacts with GSH to generate GSSG, traditional chemical catalysis and energy storage to neoteric cat-
which can be reduced back to GSH by the GR/NADPH system. alytic biomedicine. Both in vitro and in vivo experiments verified
Besides GSH, other small molecules containing thiol groups that V2C MXenzyme not only possessed desirable biocompat-
(–SH), including cysteine, can be employed as thiol cofactors, ibility but also exhibited impressive ROS-scavenging capability to
which probably accounts for the TPx-like activity of V2C protect cell components against oxidative stress through catalytic
MXenzyme. reactions. Taken together, our MXenzyme is acknowledged as a
Finally, for the underlying mechanism of V2C MXenzyme as valuable toolkit for the specific utilization in multifarious
POD mimetics, in V2C MXene structural model, the V atom is inflammation and neurodegeneration treatment.
supposed to act as Lewis acid site, but the bond pair of electrons
for bridging oxygen atoms behaves as Lewis base sites, in which Methods
the nucleophilic addition reactions of oxygen happen. Subse- Materials and reagents. Layered ternary vanadium aluminum carbide (V2AlC,
quently, the V2C MXene is supposed to react with H2O2 to form 200 mesh powders with 98% metals basis) was purchased from Forsman Scientific
Co., Inc. (Beijing, China). TPAOH (40%), 5,5-dimethyl-1-pyrroline-N-oxide
an intermediate V-peroxo (P1) species (Supplementary Fig. 46), (DMPO), buthionine sulfoximine (BSO), trichloroacetic acid (TCA), guanidine
and then the TMB substrate binds to the V-peroxo complexes via hydrochloride, and TMB were obtained from Adamas-beta Inc. (Shanghai, China).
nucleophilic attack to form P2, thus allowing the oxidation Hydrogen peroxide (H2O2), hydrochloric acid (HCl), and HF were purchased from
reaction of TMB to form the TMB*+ species. Because H2O2 is a Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Cysteine, 2-
two-electron oxidant, another TMB molecule is required for V2C monochlorodimedone (MCD), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and 1-
methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) were obtained from Aladin
MXenzyme regeneration inducing TMBox formation. Ltd. (Shanghai, China). Polyvinyl alcohol (PVA, 87-90% hydrolyzed), bovine
serum albumin (BSA), Triton X-100, phorbol 12-myristate 13-acetate (PMA), LPS,
Rhodamine B (RB), amiloride, chlorpromazine, nystatin, and hematoxylin and
Discussion eosin (H&E) were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd.
In this work, we demonstrated the specific capability of 2D V2C (Shanghai, China). Roswell Park Memorial Institute (RPMI) 1640 medium were
MXenzyme to serve as robust multifunctional inorganic analogs obtained from Hyclone Laboratories (Logan, Utah, USA). Calcein AM and pro-
of SOD, CAT, POD, TPx, GPx, and HPO, mimicking intracellular pidium iodide (PI) were both purchased from Shanghai Hongmao Biotechnology
Co., Ltd. (Shanghai, China). ROS assay kit, glutathione peroxidase (GPx) assay kit,
antioxidant defense system against ROS-mediated critical oxida- total antioxidant capacity (TAC) assay kit, cell counting kit-8 (CCK-8) assay kit,
tive damage (e.g., protein carbonylation, lipid peroxidation, and glutathione (GSH)/oxidized glutathione (GSSG) assay kit, 2-(4-Amidinophenyl)-6-
DNA damages), which extends their biomedical use from indolecarbamidine dihydrochloride (DAPI) and radio-immunoprecipitation assay
NATURE COMMUNICATIONS | (2021)12:2203 | https://doi.org/10.1038/s41467-021-22278-x | www.nature.com/naturecommunications 11You can also read