22 COVID-19 Update Week of April 19, 2021 - Prepared by: Toni P. Brown Chief Administrative Officer Delaware River Port Authority April 18, 2021
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
COVID-19 Update Week of April 19, 2021
Prepared by: Toni P. Brown
Chief Administrative Officer
Delaware River Port Authority
April 18, 2021
22▪ COVID-19 Virus Tracker - Weekly global, national, and state stats
▪ EUA Vaccine Recap
▪ Johnson & Johnson Vaccine
Clinical Trial Data
The Problem
The Temporary Pause
Dr. Leana Wen Answers Frequently Asked Questions
▪ Additional Information about the J&J Vaccine
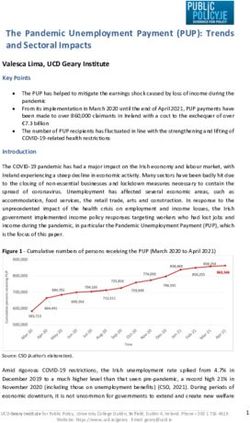
4COVID-19 Virus Tracker Infections and Deaths Statistics about the coronavirus (COVID-19) are constantly evolving. Each week I provide an update on statistics worldwide, in the U.S., and in New Jersey and Pennsylvania. The data presented are based on approximate numbers.
140 million
Confirmed COVID-19 Cases Worldwide
3.01 million
COVID-19-Related Deaths Worldwide
2.14%
Rate of COVID-19-Related Deaths Worldwide
6
Data is approximate as of 4/18/202131.6 million
Confirmed COVID-19 Cases in the U.S.
566,000
COVID-19-Related Deaths in the U.S.
1.79%
Rate of COVID-19-Related Deaths in the U.S.
7 Data is approximate as of 4/18/20211.1 million
Confirmed COVID-19 Cases in the NJ
25,681
COVID-19-Related Deaths in the NJ
2.33%
Rate of COVID-19-Related Deaths in the NJ
8 Data is approximate as of 4/18/20211.08 million
Confirmed COVID-19 Cases in the PA
25,459
COVID-19-Related Deaths in the PA
2.54%
Rate of COVID-19-Related Deaths in the PA
9 Data is approximate as of 4/18/2021Vaccines Status of EUAs Granted
COVID-19 Vaccines Granted
Emergency Use Authorization (EUA) by
U.S. Food and Drug Administration (FDA)
▸ United States of America
▹ EUA granted 12/11/2020
■ Pfizer-BioNTech
■ 16 years and older
■ mRNA vaccine
▹ EUA granted 12/17/2020
■ Moderna (in partnership with NIAID)
■ 18 years and older
■ mRNA vaccine
11
■ Health Digest: Common Side Effects of the Moderna Vaccine ExplainedEmergency Use Authorization (EUA)
by FDA
▸ United States of America
▹ EUA granted 2/27/2021
■ Janssen (Johnson & Johnson)
■ 18 years and older
■ Adenoviral vector vaccine READ: NYTimes - An excellent primer on
adenovirus vector vaccines
■ FORBES: Everything You Need To Know About Johnson & Johnson’s
COVID-19 Vaccine
12Oxford-AstraZeneca’s Vaccine
Status: In the Pipeline
▸ Oxford University and AstraZeneca (AZD1222)
▹ Viral Vector Vaccine
▹ A specially engineered virus delivers genetic instructions to human cells to make the
spike protein jutting out from the new coronavirus’s surface.
▹ AstraZeneca pledged the vaccine would cost just a few dollars per dose and be sold
without making a profit. ($4.00 per dose)
▹ Compare to Pfizer’s vaccine costs per dose: $18.40 - $19.50.
13Oxford-AstraZeneca’s Vaccine
Status: In the Pipeline
▸ Oxford University and AstraZeneca (AZD1222)
▹ Data from clinical trial in the United States, Chile and Peru
■ Trials included 32,449 adult participants in all age groups, most of them in the
United States
■ The volunteers received two standard doses of the Oxford-AstraZeneca vaccine,
or a placebo, at a four-week interval
■ Scientists said the data shows that the vaccine is 79% effective against
symptomatic COVID-19, and 100% effective against severe illness
14Vaccine Distribution & Administration Progress to Date - as of April 18, 2021 Biden/Harris Goal: 100 million shots in arms in 100 days The Biden administration has until the end of the day April 29 to reach the target. Approximately 24.8% of the total U.S. population– approximately 82.4 million people -- is now fully vaccinated and millions are getting a shot every day. Herd immunity is estimated to be about 70% of the population.
Vaccine Tracker – United States
As of April 18, 2021 Based on Data from the Centers for Disease Control and Prevention
Overall US COVID-19 Vaccine Distribution and Administration
• 264,499,715 million doses have been delivered
• 205,871,913 million doses have been administered
• Number of people fully vaccinated: 82,471,151
• Pfizer-BioNTech administered– 107,083,884 million
• Moderna administered – 90,718,986
• Janssen (Johnson & Johnson) administered – 7,902,746
• Not identified – 166,297
16Vaccine Tracker – New Jersey
Source: New Jersey Department of Health as of April 18,2021
New Jersey
▪ 5,934,148 Doses Administered as of 4/17/2021
▪ People with at least one vaccine dose – 3,745,150
▪ Fully vaccinated people – 2,428,796
▪ Percentage of population fully vaccinated – 29.3%
▪ Vaccine Brand
▪ Moderna – 44%
▪ Pfizer-BioNTech – 52%
▪ Janssen (Johnson & Johnson) - 4%
17Vaccine Tracker – New Jersey
Source: New Jersey Department of Health as of April 17, 2021
New Jersey Gender
▪ By Race/Ethnicity Female – 55%
▪ Black – 6% Male – 45% (up 1%)
▪ Hispanic/Latinix – 10% (up 2%)
▪ Asian – 9%
▪ Other – 10%
▪ Unknown – 9%
▪ White – 57%
18 ▪ Percentage equals 101%; data taken from NJ DOT websiteVaccine Tracker – Pennsylvania
Source: Our World Data as of April 17,2021
Pennsylvania
▪ Approximately 8.39 million doses Administered as of 4/17/2021
▪ Fully vaccinated people – Approximately 3.28 million
▪ Percentage of population fully vaccinated – 25.6%
19Vaccine Tracker – Philadelphia
Source: City of Philadelphia Vaccination Dashboard as of 4/16/2021Depa
Philadelphia
▪ Fully vaccinated – 438,422 (increase of 75,233 from last week)
▪ Individuals receiving at least one (1) dose – 649,584
▪ Pfizer-BioNTech – 656,715
▪ Moderna – 374,973
▪ Johnson & Johnson – 28,159
▪ Grand Total = 1,059,847
20Johnson & Johnson Vaccine
Temporary Pause
The CDC and FDA have recommended pausing the use of
millions of J&J doses “out of an abundance of caution” while
they investigate reports of rare and severe blood clots in a
handful of women who received the shot.
According to health experts, the ‘temporary pause’ is evidence that public
health and oversight institutions are working exactly as they should.Johnson & Johnson Vaccine
What did the large trial data reveal before the EUA?
▸ Trial Data – 45,000 people were fully enrolled in clinical trial
■ The Food and Drug Administration authorized J&J’s single-dose vaccine for
emergency use in late March.
■ At the time it noted concerns about blood clots in some trial volunteers but
said there was not enough data to establish a link with the vaccine.
■ Although many of the people who developed clots in the trial had underlying
health issues, one 25-year-old man with no prior conditions had a severe case.
■ FDA advised providers to monitor whether more blood clots occurred in the
general populations to learn more about potential risks.
22Johnson & Johnson Vaccine
Temporary Pause – What led to the pause?
▸ Six (6) Known Cases
■ So far, more than 7 million Americans have received the J&J vaccine.
■ What happened? Six women, between the ages of 18-48, developed a rare but severe
blood clot condition called cerebral venous sinus thrombosis (CVST). The brain blood clot,
was combined with thrombocytopenia, or low platelet counts in the blood, after their J&J
vaccination. Three women also had large, dangerous clots in other parts of their body as
well as in the brain.
■ The women developed symptoms, most often headaches, six to 13 days after vaccination.
But the symptoms were not necessarily indicative of a serious problem.
■ The CDC wants clinicians to be aware of the issue, especially because the traditional
treatment for clotting — the blood thinner heparin — could actually make this specific
condition worse. CDC wants doctors everywhere to “plan for proper recognition and
23 management due to the unique treatment required.”Johnson & Johnson Vaccine
Temporary Pause – What led to the pause?
▸ Six (6) Known Cases
■ The CDC panel, known as the Advisory Committee on Immunization Practices (ACIP) is not
only reviewing the six reported cases of clots among J&J recipients but discussing whether
risk of using the shot outweighs the benefit.
■ Neither the company nor the regulators know whether any of the known cases of clotting
involved people who were pregnant or postpartum, but one person was taking oral
contraceptives.
24Johnson & Johnson Vaccine
Pause to continue as CDC panel postpones decision
■ Members of the CDC’s ACIA panel said they did not feel comfortable making a
decision about whether to continue vaccinations because there was not
enough evidence about the patients who experienced the serious but rare side
effects.
■ Panel members said they wanted more information about the people who may
be most at risk for blood clots such as age and gender.
■ The panel did not set a date for when they will meet again, but it could be in
the next week to 10 days. There is also a regularly scheduled meeting on May 5.
■ The Advisory Committee's recommendations are non-binding, but health
officials have indicated they will use the panel to help inform their final
decision about the shot.
25Johnson & Johnson Vaccine
Pause to continue as CDC panel postpones decision
■ Top Biden administration officials said they expect the pause will last days or
weeks, not months.
■ But it's not clear what affect the lack of a vote from the committee will have on
that timeline, nor is it clear what level of evidence the committee members
want before making any recommendations.
■ While most of the committee members who spoke seemed to favor the
pause and were convinced it would only reinforce confidence in the
vaccine, some expressed frustration at the lack of action, as well as about
the impact a continued pause will have on vulnerable communities.
26Johnson & Johnson Vaccine
Pause to continue as CDC panel postpones decision
■ "We are in a situation where not making a decision is tantamount to
making a decision," said Nirav Shah, president of the Association of State
and Territorial Health Officials and director of Maine's public health
agency.
■ He urged the committee to understand the equity concerns that could
arise from delaying use of the vaccine any further.
■ Still, other members of the committee noted that the U.S. has two
alternative vaccines in the Moderna and Pfizer-BioNTech products that
can be used as a backstop. Those shots use mRNA technology, while
Johnson & Johnson's vaccine is based on an adenovirus.
■ The J&J vaccine is only a small part of the U.S. vaccination campaign,
making up less than 5% of the total doses administered so far.
27What should people who just got the Johnson & Johnson
vaccine do?
Dr. Anthony Fauci’s advice:
“Well, it depends on when they got it. It appears that this adverse event
occurs between six days and 13 days. So, if you've had it a month or two
ago, I think you really don't need to worry about anything. If you are in
the timeframe of within a week or two of having gotten vaccinated,
remember one thing: This is a very rare event. It's less than one in a
million. Having said that, you still wanna be alert to some symptoms,
such as severe headache, some difficulty in movement, or some chest
discomfort and difficulty breathing.”
28Johnson & Johnson FAQs about the Pause Dr. Leana Wen, an emergency physician, visiting professor of health policy and management at the George Washington University Milken Institute School of Public Health, and CNN Medical Analyst, received the Johnson & Johnson vaccine as a volunteer participant in a clinical trial. In the slides that follow, she addresses concerns over the pause on the J&J vaccine rollout.
Johnson & Johnson Vaccine
FAQs
What is a pause?
Dr. Wen: The federal health authorities were very specific that this is a recommendation
for a pause, not a requirement to stop the Johnson & Johnson vaccine rollout. There's a big
difference here. The pause is to say there is a possible safety concern, and the FDA and CDC
want time to investigate the concern. In the meantime, they are asking mass vaccination
sites, pharmacies and doctors' offices to hold off on giving this vaccine.
What's going to happen to patients who are currently scheduled to get the vaccine?
Dr. Wen: Patients currently scheduled for the Johnson & Johnson vaccine are probably
going to receive another vaccine instead.
30Johnson & Johnson Vaccine
FAQs
What would you say to those people who have taken the Johnson & Johnson
vaccine?
Dr. Wen: I wasn't concerned for myself, but I was concerned for my patients and the public
because I knew that so many people would be worried. My advice to them is the advice I'm
following myself, as someone in this group: First, know that the risk of these very rare
blood clots is very, very low. It's an extremely unlikely event.
Still, it's important to know what to watch out for, and when. If you just got the vaccine and
now are having fevers, headache and muscle aches, that's the normal and expected
reaction from your body producing an immune response. I wouldn't worry about symptoms
in the initial 12 to 24 hours after getting the vaccine. It's also very unlikely that the vaccine
will cause a delayed reaction a month after the inoculation.
31Johnson & Johnson Vaccine
FAQs
What would you say to those people who have taken the Johnson & Johnson
vaccine?
Dr. Wen: If you are within three weeks of the Johnson & Johnson vaccine, look out for new
and different symptoms. What I mean is that if you normally have migraines and this is your
normal migraine, that's not a problem, but if you usually don't have headaches and now you
have a severe, unrelenting headache, you should call your doctor. Also call your medical
provider if you have shortness of breath, abdominal or chest pain, numbness or weakness in
arms or legs, or swelling in one of your arms or legs. Make sure to mention that you
received the vaccine and bring your CDC card that notes exactly when you got the vaccine
and from where.
32Johnson & Johnson Vaccine
FAQs
Some people have said this is an overreaction. After all, it's six cases, out of
nearly 7 million people who have received the Johnson & Johnson vaccine, and
it's not clear that the vaccine caused the clot. What's your view on this?
Dr. Wen: I think the FDA and CDC made the right decision. Here's why.
First, we're not talking about the run-of-the-mill blood clot here. We're talking
about a very rare and very serious blood clot in the brain. It's even more rare
when it's accompanied by this low platelet condition.
Second, there are real implications on treatment. The normal treatment for
blood clots is an anticoagulant drug called heparin. Heparin, if administered to
these patients, could worsen the outcome. It was really important to get the
word out to clinicians on looking out for patients with the combination of CVST
and thrombocytopenia and treating them accordingly.
33Johnson & Johnson Vaccine
FAQs
Third, it may be that the six women who have been diagnosed with the rare
conditions have something in common. Maybe they have a similar underlying
condition. If that's the case, patients with that kind of condition can be advised
to avoid this vaccine and take one of the other two vaccines, from Pfizer-
BioNTech or Moderna. When there is a possible side effect that's this severe, it
makes sense to take it seriously, alert the public, fully investigate and come up
with recommendations.
34Johnson & Johnson Vaccine
FAQs
What are next steps with the FDA and CDC?
Dr. Wen: They are both continuing their investigations. The agencies will look into the
known cases to identify commonalities and to look for causality. They will also see if there
are other cases that may not be reported and could be related to this vaccine.
It's possible that a recommendation could come out that would release the overall pause
but still have a pause on a specific subgroup, for example, people under a certain age. I
expect the recommendation to come out in a matter of days.
35Johnson & Johnson Vaccine
FAQs
What do you say to people who are concerned about this kind of pause
worsening vaccine hesitancy?
Dr. Wen: I think people should take away exactly the opposite message. They should see
that the FDA and CDC are prioritizing safety first and foremost — that they're willing to
pause a vaccine rollout to investigate a possible side effect that's literally one in a million!
They are proceeding in a transparent, thorough and careful way.
To me, this says that we can trust our federal health authorities to do a fair and timely
investigation of this vaccine. It also says that we should really trust the safety of the two
other vaccines we have.
36Johnson & Johnson Vaccine
Perspective
▪ The fact that federal health officials would pause the Johnson & Johnson
vaccine to look into a one in a million clotting disorder should give
confidence that they are investigating thoroughly & carefully—and, I hope,
bolster public confidence in the Pfizer & Moderna vaccines.
pic.twitter.com/PtWOV6Ih4z
Leana Wen, M.D. (@DrLeanaWen) April 13, 2021
37Johnson & Johnson Vaccine
Additional Information
▪ Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine
▪ Imbedded Video: Dr. Fauci on what the Johnson & Johnson vaccine reactions could mean for
women
▪ The Facts on the Recommended J&J Vaccine ‘Pause’
▪ Press Secretary Jen Psaki, Dr. Fauci White House Press Conference Transcript April 13: Pause
on Johnson & Johnson Vaccine
▪ Blood clots and COVID-19 vaccines: How scientists are starting to unravel the rare side effect of
the J&J and AstraZeneca vaccines
▪ Johns Hopkins Medicine: What is Cerebral Venous Sinus Thrombosis (CVST)?
▪ Cleveland Clinic: What is Thrombocytopenia?
39Thanks
Questions or suggestions for
future updates?
You can find me at:
tpbrown@drpa.org
40Additional Sources
▸ https://www.washingtonpost.com/graphics/2020/health/covid-vaccine-states-distribution-
doses/?itid=lb_coronavirus-what-you-need-to-read_5
▸ https://covid.cdc.gov/covid-data-tracker/#vaccinations/
▸ https://www.cnn.com/2021/04/15/health/johnson-and-johnson-vaccine-concerns-
wellness/index.html
▸ https://thehill.com/policy/healthcare/548301-johnson-johnson-vaccine-pause-to-continue-
as-cdc-postpones-decision
41You can also read