Pathogenesis of NASH and Promising Natural Products
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Available online at www.sciencedirect.com
Chinese Journal of Natural Medicines 2021, 19(1): 12-27
doi: 10.1016/S1875-5364(21)60002-X
•Review•
Pathogenesis of NASH and Promising Natural Products
LENG Ying-Rong, ZHANG Mei-Hui, LUO Jian-Guang*, ZHANG Hao*
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Bioactive Natural Product Research, School of Tradi-
tional Chinese Pharmacy, China Pharmaceutical University, Nanjing 210009, China
Available online 20 Jan., 2021
[ABSTRACT] Nonalcoholic steatohepatitis (NASH) is a common clinical condition that can lead to advanced liver diseases. The
mechanism of the diaease progression, which is lacking effective therapy, remains obsure. Therefore, there is a need to understand the
pathogenic mechanisms responsible for disease development and progression in order to develop innovative therapies. To accomplish
this goal, experimental animal models that recapitulate the human disease are necessary. Currently, an increasing number of studies
have focused on natural constituents from medicinal plants which have been emerged as a new hope for NASH. This review summar-
ized the pathogenesis of NASH, animal models commonly used, and the promising targets for therapeutics. We also reviewed the nat-
ural constituents as potential NASH therapeutic agents.
[KEY WORDS] NASH; Natural medicine; Animal model; Target; Pathogenesis
[CLC Number] R965; R284 [Document code] A [Article ID] 2095-6975(2021)01-0012-16
Introduction steatosis, and liver biopsy remains the gold standard for the
diagnosis of NAFLD and to distinguish steatosis from
Nonalcoholic fatty liver disease (NAFLD) is the most
NASH. However, diagnostic accuracy can be improved by
frequent liver disease worldwide, and is commonly associ-
combining blood biomarkers and imaging tools [6]. Albeit the
ated with the metabolic syndrome. Within the general popula-
substantial progress of knowledge about pathophysiology and
tion, the overall global prevalence of NAFLD came up to
targets has been made, substantial challenges exist for the
25% though substantial variability was noted across geo-
therapeutic methods for NASH. Due to the complicated
graphic regions [peak prevalence in the Middle East (31.8%)
pathogenesis and individual heterogeneity in NASH, there
and South America (30.4%), while the lowest rates in Africa
(13.5%)] [1]. In China, NAFLD, whose prevalence is approx- has been no approved agents to date for the treatment of
imately 29.2%, has become the most prevalent liver disor- NASH regardless of that many compounds are under clinical
der [2]. NAFLD is defined by excess lipid accumulation in the evaluation, which has been extensively reviewed [7]. In addi-
liver of a patient in the absence of excessive alcohol intake [3]. tion, animal models are vital for search of novel targets and
The spectrum of NAFLD ranges from simple steatosis to better understanding of pathogenesis. Suitable models that re-
NASH ,fibrosis and cirrhosis, and NAFLD may even pro- semble human NASH can help patients stratification and
gress to hepatocellular carcinoma without apparent cirrhosis. evaluation of therapeutic outcomes in the development.
According to clinical experiences, NASH is characterized by Traditional Chinese medicines (TCMs) are abundant
hepatocellular lipid accumulation (steatosis) along with lobu- sources of biologically active substances that can be applied
lar inflammation, hepatocellular ballooning, and often associ- to prevent human diseases. Currently, an increasing number
ated with fibrosis [4-5]. Currently, no highly sensitive and spe- of studies have focused on natural products, which showed
cific tests are available to differentiate NASH from simple potential effects against NAFLD [8]. In China, the majority of
clinical trials have focused on investigating the effects of
herbal extracts and natural products on NAFLD [9]. Thus, nat-
[Received on] 23-Nov.-2020
[Research funding] This work was supported by the National Natur- ural constituents are promising candidate drugs for NAFLD
al Science Foundation of China (No. 81872889), and the Drug Innov- treatment. In this review, we briefly summarized the com-
ation Major Project (Nos. 2018ZX09711-001-007 and 2018ZX09735 monly used animal models, and the promising targets for
002-003).
therapeutics. Subsequently, we systematically review the role
[*Corresponding author] E-mail: luojg@cpu.edu.cn (LUO Jian-
Guang); zhanghao@cpu.edu.cn (ZHANG Hao) of natural constituents for the treatment of NASH, which may
These authors have no conflict of interest to declare. provide potential directions.
– 12 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
Pathogenesis of NASH and Main Targets for the and adaptive inflammatory responses [17]. TLR4 functions as a
NASH Treatment LPS sensor, and the activation of which will recruit adaptor
protein myeloid differentiation primary response gene 88
The complex pathogenesis makes it hard to demonstrate (MyD88) and activate downstream NF-κB cascades [18]. Mice
the clear mechanism of NASH, whose initiation addresses
deficient in TLR4 exhibit low expression of proinflammatory
multiple events. The pathogenesis of NASH is controversial
cytokines even when these mice were fed a NASH-inducing
for that there has been two widely recognized theories for the
diet [19]. JKB-121, a TLR4 antagonist, whose phase 2 trial for
explanation of NASH, namely “two hit ” along with “ mul-
the treatment of NASH is ongoing (NCT02442687). In re-
tiple hit ”. “Two hit ” theory suggests that in the context of
cent years, NOD-like receptor protein 3 (NLRP3) inflamma-
simple hepatosteatosis alone, the second ‘hit’ which is re-
some, consisting of several sensor and signaling proteins, has
sponsible for liver injury was required for the development of
been identified as another trigger for liver inflammation in
NASH [10]. However, this view is outdated and “multiple hit”
NAFLD [20]. The signaling proteins combine to form active
in which pathogenetic events occur simultaneously is more
caspase 1 from procaspase 1. Caspase 1 is necessary for the
favored. All the events that lead to the development of NASH
secretion of interleukin (IL)-1β and IL-18 [21]. It has been
provide potential targets, including liver insulin resistance,
proved that pharmaceutical blockade of NLRP3 activation ef-
lipotoxicity, oxidative stress (including reactive oxygen spe-
fectively improve NAFLD pathology and fibrosis severity [20].
cies), endoplasmic reticulum (ER) stress, mitochondrial dys-
Chemokine receptors (CCR) are G-protein coupled re-
function, adipose tissue dysfunction and dysregulated innate
immunity, cytokine secretion and the gut-liver axis [11-12], ceptors (GPCRs) that combine with chemokine ligands to
which is affected by microbiota dysbiosis. In this review we trigger downstream cascades essential in facilitating basal
primarily focus on several processes associated with cardinal and inflammatory leukocyte migration [22]. CCR2 and CCR5
signs including inflammation, fibrosis, carbohydrate metabol- are the most characterized chemokine receptors that involved
ism and relevant targets under evaluation (Table 1). in the progression of NASH and fibrosis. Inhibition of CCR2-
Inflammatory cells and cytokines have been reported to CCR5 axis reversed short-term fibrosis in a clinical trial of
participate in NASH pathogenesis. Apoptosis signaling NASH [23]. It was shown in mice that overexpression of
kinase-1 (ASK-1)-JNK, MAP kinases, ERK, and NF-κB are CCR2 ligand, chemokine ligand 2 (CCL2), leads to adipose
potent mediators of inflammation and thus potential targets tissue inflammation and hepatic steatosis [24]. In rodent mod-
for therapy. ASK1 is a serine/threonine protein kinase in the els of diet-induced NASH, treatment with a modified version
mitogen-activated protein kinase kinase kinase (MAP3K) en- of CCL5 that functions as an antagonist (Met-CCL5) or the
zyme family and acts upstream of Jun N-terminal kinase small-molecule CCR5 antagonist maraviroc (a US Food and
(JNK) and p38 [13]. Multiple elements, such as tumor necrosis Drug administration (FDA)-approved inhibitor of CCR5-me-
factor α (TNFα), lipopolysaccharide (LPS), ER stress, calci- diated entry of HIV into immune cells) ameliorated NASH
um influx, and oxidative stresses are capable to activate and fibrosis [25-26]. Cenicriviroc, a dual CCR2 and CCR5 ant-
ASK1. ASK1 then phosphorylates and activates an intermedi- agonist with nanomolar potency, had potent anti-inflammat-
ate kinase (MAPK kinase 4), which in turn activates the JNK ory and anti-fibrotic activity in mouse models of NASH [27].
and p38 pathways in hepatic cells. These pathways lead to Metabolic syndrome (MetS), including increased waist
apoptosis, inflammation, and fibrosis [14]. Studies with rodent circumference (i.e., obesity), hyperglycemia, dyslipidemia
models have supported the potential utility for ASK1 inhibit- and systemic hypertension, is the strongest risk factor for
ors as a treatment for NASH [15]. Of note, the antagonism of NAFLD and NASH [28]. The metabolites induce hepatocellu-
ASK-1 ameliorated NASH and alleviated fibrosis in some pa- lar stress, injury and death, leading to fibrogenesis and gen-
tients in a short-term clinical trial [16]. omic instability that predispose to end-stage liver diseases.
Toll-like receptor 4 (TLR4) signaling has also been im- The availability of metabolic targets for therapeutic strategies
plicated in the pathogenesis of NASH by mediating innate relies on reducing metabolic substrate delivery to the liver or
Table 1 The review of main targets for the NASH treatment
Name Function Relevant agents
ASK1 regulating inflammation and fibrogenesis ASK1 antagonist: selonsertib (Ph 3)
TLR4 regulating inflammation, function as a LPS sensor TLR4 antagonist: JKB-121 (Ph 2)
regulating inflammation by controlling basal and Dual CCR2 and CCR5 antagonist: cencriviroc (Ph 3) CCR5
CCR2/5
inflammatory leukocyte trafficking antagonist: Met-CCL5, maraviroc (FDA approved)
regulating fatty acid and glucose metabolism, FGF21 analogues: BMS-986036 (Ph 2); AKR-001 (Ph 2) PEGylated
FGF21
enhance insulin signaling FGF21 and Fc-FGF21(RG) (Ph 2a)
ACC regulating fatty acid synthesis ACC antagonist:PF-05221304 (Ph 2)
regulating bile acid synthesis and multiple metabolic
FXR FXR agonist: obeticholic acid (OCA) (Ph 2a)
pathways
– 13 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
facilitating its safe disposal. Hence, there has been an explos- treatment of mice with an intestine-selective FXR antagonist
ive interest in targets correlating with lipid metabolism in alleviated metabolic function in diet-induced and genetic
NASH progression. obesity [44].
Fibroblast growth factors (FGF) are peptide hormones
Animal Models Used for NASH
that play important roles in NASH development involving
fatty acid and glucose metabolism [29]. FGF19 and FGF21, the The progress of drug development for NASH relies
most widely investigated members, together with their recept- primarily on available animal models, which can mimic the
ors are promising targets for NASH. FGF21 transcription is real situation of human NASH. It is a pity that no single mod-
upregulated by ER stress, SIRTI and transcription factors el perfectly displaying the full spectrum of the NASH patho-
such as PPARα and PPARγ [14]. FGF21 augments insulin physiology. Herein we focus on the animal models highly ap-
sensitivity in adipose and hepatic tissues by increasing gluc- plicable in researches. Animal models of NASH can be
ose transporter type 1 (GLUT1) expression, suppressing hep- widely categorized as dietary, genetic, toxins-based, and
atic gluconeogenesis, inducing insulin signaling in adipo- combination models (Table 2).
cytes [30], and reduces steroyl-CoA response element binding Dietary models
protein-1c (SREBP1c) mediated lipogenesis in the liver [31]. Large portion of animal models are aimed at triggering
Several analogs of FGF21 have been advanced into clinical liver damage. Therefore, diets deficient in nutrition are theor-
development [7]. BMS-986036 (pegbelfermin, BMS) was etically available for establishing models of NASH. The me-
tested in a 16-week phase 2 trial in NASH patients. Adminis- thionine and choline deficient (MCD) diet lack two essential
tration of the peptide by subcutaneous injection once daily factors, namely methionine and choline, whereas it’s rich in
resulted in dramatic reductions in liver fat at week 16 com- sucrose and fat (40% sucrose, 10% fat). Normal mice fed
pared to placebo (ClinicalTrials.gov identifier NCT0241- MCD and choline-deficient l-amino acid (CDAA)-defined
3372). Acetyl-CoA carboxylase (ACC) is a biotin carboxyl- diet develop a histological appearance similar to NASH with
ase that plays a key role in regulating fatty acid synthesis [32]. fat, inflammation, and fibrosis, but they fail to recapitulate
There are two isoforms of ACC (ACC1 and ACC2) that have the accompanying symptoms of obesity and insulin resist-
distinct tissue locations and metabolic functions [32-33]. ACC1 ance, rather they will cause body weight loss. Hence, mice on
is located in the liver, adipose tissue, and mammary gland, CDAA or MCD diet bears little resemblance to the etiology
whereas ACC2 is primarily expressed in skeletal and heart and metabolic features of human NASH. In addition, the
muscle. ACC antagonism improved steatosis, inflammation, CDAA diet often takes longer to elicit the histological liver
and fibrosis in both animal models and human derived cells changes than the MCD diet does [45-47].
in vitro systems [34]. A phase 2a clinical trial results showed The strong association between obesity and NAFLD has
that ACC inhibitor PF-05221304 improved steatosis and liv- spurred the development of various diet-induced obesity
er injury in NAFLD patients (ClinicalTrials.gov identifier (DIO) models. The high-fat diet (HFD) induces liver injury
NCT03248882) [14]. by providing saturated fats, which supply 60% caloric value
Farnesoid X receptor (FXR) is a nuclear hormone recept- of the diet, results in slight pathogenic outcomes but practic-
or as well as a bile-acid sensor, regulating bile acid levels ally no inflammation or severe liver injury. The mice fed on
through modulating multiple pathways including bile acid HFD progress to obesity and insulin resistance after about 9
synthesis, de novo lipogenesis, and glucose metabolism [35]. In weeks with perisinusoidal hepatic fibrosis. HFD does not de-
the liver, FXR agonists enhance insulin sensitivity [36], in- velop hepatic inflammation until approximately 19 weeks on
crease triglyceride clearance and mitochondrial fatty acid β- the diet [46, 48-49].
oxidation, and suppress lipogenic gene transcription [37]. In ro- The DIO models with macronutrient composition resem-
dent models of diet-induced NASH, FXR agonists prevent bling that of obese humans are popular mouse model of
the development of NASH and promoted the resolution of NASH; however, the distinction between strains and species
steatohepatitis and fibrosis [38]. Obeticholic acid (OCA) is a must also be taken into consideration. For instance, the
semi-synthetic derivative of chenodeoxycholic acid with pi- choline-deficient l- amino-defined high fat diet (CDA-HFD)
comolar agonistic activity on FXR [39]. A small randomized is able to induce an enlarged fatty liver with fibrosis in 6
trial in type 2 diabetic patients with NAFLD showed an im- weeks in C57BL/6J mice while gaining or maintaining body
provement in insulin sensitivity as measured by euglycemic weight, yet in a different strain, A/J mice, a steady decline in
clamp, a modest but dose-related weight loss, and a reduc- body weight is observed [50].
tion in ALT levels [40]. Nevertheless, appeal of small mo- Genetic models
lecule agonists targeting FXR is the most advanced modality Genetic alterations can induce energy consumption, and
and is a highly promising strategy for treating NASH, either fatty acids are delivered substantially to the liver, where ulti-
as a standalone therapy or in combination with other mately excessive lipids accumulate. The commonly used
drugs [14]. Controversially, several studies have suggested that models are the leptin (ob/ob)-deficient, leptin-receptor
depletion or antagonism of FXR, specifically in the intestine, (db/db)-deficient mice, foz/foz mice (deficient in the Al-
may have therapeutic potential in NASH [41-43]. Interestingly, strom syndrome 1 gene), the Zucker (fa/fa) rat, and several
– 14 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
Table 2 Commonly used animal models of NASH
Model Advantages Disadvantages
Dietary
Animals lose weight and are not insulin resistant,
Methionine and choline deficiency The histologic appearance in 2−4 weeks resembles
with lack of concordance of the liver transcriptome
(MCD) human NASH
with human NASH
The histologic appearance of lipid accumulation, The histologic changes require for long time to
Choline-deficient L-amino-defined
inflammation, liver fibrosis, and it takes shorter to develop; the animals don’t have change in weight
diet (CDAA)
attain these changes than CDAA diet and peripheral insulin sensitivity
It can replicate the altered metabolic parameters
High-fat diet (HFD) The hepatic pathogenic outcome is not severe
observed in human fatty liver disease
High-fat, fructose and cholesterol Animal develop both histologic and metabolic
Animals exhibit no cirrhosis
(AMLN diet) features of human NASH with diffuse fibrosis
Toxins/Diet-based
Carbon tetrachloride (CCl4)
Weight loss. It's most likely to progress to cirrhosis
Commonly used for inducing fibrosis; The fibrosis
Thioacetamide (TAA) and HCC, it does not mirror the etiology and
induction period is short
natural history of NASH
streptozotocin (STZ)
Genetic
ob/ob mice are resistant to fibrosis without a second
Animals develop severe insulin resistance and
Both leptin-deficient (ob/ob) hit. Unlike this model, humans with NASH have
steatosis
normal or elevated serum leptin
Severe glucose intolerance, obesity and hepatic db/db mice do not develop NASH or fibrosis
Leptin receptor-deficient (db/db) steatosis; susceptible to fibrosis with a second insult without a second insult; develop fibrosis with
(e.g., MCD or high-fat diet) addition of MCD diet
Severe hyperphagia, insulin resistance and obesity on
The Zucker (fa/fa) rat exhibiting modest fibrosis
HFD, with hepatic steatosis
Histologically exhibiting extensive immune
The mice do not develop to steatohepatitis without
MUP-uPA transgenic infiltration with ballooning hepatocytes. Pathological
a second insult (e.g. MCD diet)
features similar to human NASH
Liver steatosis progresses to steatohepatitis with
insulin hypersensitivity and decreased body fat
PTEN KO ballooned hepatocytes, Mallory’s hyaline, lobular
mass
inflammation; develop benign liver adenomas or HCC
Metabolic syndrome with hepatic lipid accumulation, Liver steatosis in these mice does not
KK-Ay/a
susceptible to MCD-induced steatohepatitis spontaneously advance to steatohepatitis
It can show the common characteristics of human It fails to recapitulate aspects of metabolic
PTEN−/−
NASH, together exhibits the disease progress syndrome, such as obesity and insulin resistance
Develop severe steatosis when subjected to fasting for It does not develop fatty liver under normal feeding
PPARα−/−
24 h or HFD conditions
Combined
Animals exhibit type 1 diabetes and are lean,
different from NASH phenotype; mild fibrosis,
STAM (STZ + HFD) It progresses rapidly to histological changes
liver transcriptome is discordant from human
NASH.
Progressive steatohepatitis, fibrosis then cirrhosis and require a long course of dietary intervention to
DIAMOND mouse HCC. High transcriptomic concordance to human replicate many histologic characteristics of human
NASH NASH
transgenic or conditional knockout mice. Ob/ob mice have hepatic fibrosis or NASH without a secondary insult, such as
high phenotypic concordance to db/db mice, the phenotype is the addition of a NAFLD-inducing or NASH-inducing
characterized by obesity, hyperphagia, hyperglycaemia, in- diet [53]. Mice which are mutated or deficient in Alms1 are
sulin resistance, hyperlipidaemia and the spontaneous devel- called foz/foz mice. Like ob/ob and db/db mice, these mice
opment of fatty liver [51-52]. Both ob/ob and db/db mice have are also obese, insulin resistant and display steatosis [54-55].
the advantage of phenotypes similar to human metabolic syn- KK-Ay/a mice possess a heterozygous mutation in the
dromes; however, both models fail to spontaneously develop agouti gene. These mice are hyperphagic as a result of im-
– 15 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
paired hypothalamic food intake suppression [56]. They devel- C57BL/6J mice are given low-dose streptozotocin immedi-
op obesity and associated hyperglycemia, hyperinsulinemia, ately after birth, surviving mice are then started on HFD.
insulin resistance, and liver steatosis. Liver steatosis in these These mice develop steatohepatitis, fibrosis and HCCs in ap-
mice does not spontaneously advance to steatohepatitis, proximately 20 weeks [64]. This model recapitulates several
which, however, can be induced by additional stimulus, such important histological aspects of human NAFLD and is also
as the MCD diet [57]. In addition, Nakagawa et al. created a mo- associated with oxidative stress [66], however, it differs from
del of NASH utilizing major urinary protein (MUP)-urokin- the human state as β cell function loss is induced by strepto-
ase- type plasminogen activator (uPA) mice, which can indu- zotocin rather than a systemic, inflammatory, insulin resist-
ce hepatocyte ER stress and transient liver damage [58-59]. It has ant milieu.
been reported that HFD-fed MUP-uPA mice show substan- Another recently developed model is the Diet Induced
tial upregulation of collagen gene expression, hepatic stellate Animal Model of Non-alcoholic Fatty Liver Disease (DIA-
cells (HSCs) activation, and upregulation of fibrogenic mark- MOND) mouse, which also incorporates a high fat and
ers. In conclusion, the HFD-fed MUP-uPA model is almost fructose diet on a specific genetic strain (129S1/SvImJ and
identical to human NASH in its pathological features [60]. C57BL/6J cross) [67]. The DIAMOND mouse is able to re-
Other genetic models such as SREBP-1c transgenic capitulate all histological features of NASH including bal-
mice, and mice with global deficiencies in phosphatase and looning. Although this model better represents clinical hu-
tensin homolog (PTEN), PPAR-a, acyl-coenzyme A oxidase man NASH pathological process, it requires a long course of
(AOX), and methionine adenosyltransferase 1A (MAT1A) dietary intervention (months to years) to present many fea-
have also been studied. As previously reviewed [53], these tures of NASH.
models have limitations since they fail to display obesity.
Potential Natural Constituents for NASH Ther-
Moreover, RNA-sequencing (RNA-seq) analysis had shown
apy
that the liver gene expression profile of Pten-/- mice is quite
discordant from other mouse models of NASH [61]. Based on the reality that there have been no approved
Toxins-based models agents for the treatment of NASH, we summarize the natural
Chemically induced parenchymal liver damage and constituents which have anti-steatohepatitis activity. The
fibrosis is specifically used for studying mechanisms of hep- deeper exploration of natural compounds may be promising
atic fibrosis progression and regression. The frequently ap- direction for curing NASH. Here, we categorize them into
plied chemotoxins contain carbon tetrachloride (CCl4), thio- four subsets according to their functions in the review
acetamide (TAA), and streptozotocin (STZ). CCl4 is the most (Table 3). The potential natural compounds and the regulated
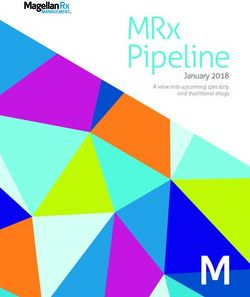
widely used toxin for inducing reproducible and predictable aspects are showed in Fig.1.
hepatic fibrosis, contributing to severe centrilobular necrosis Regulating metabolism
in hepatocytes through its metabolite trichloromethyl radi- Numerous natural compounds have been reported to reg-
cal [62]. STZ is particularly toxic to pancreatic β cells, leading ulate metabolisms (Fig. 2). Emodin (1), isolated form Rheum
to progressive loss of insulin generation, but STZ can also ha- palmatum L., has been used in the treatment of ischemic dis-
ve hyperglycemia-independent direct hepatotoxic effects [63]. ease and inflammatory disease previously [68]. Dong et al. es-
Of note, CCl4 and TAA (with or without high-fat dieting) are tablished a rodent mouse model fed with high caloric laborat-
used in adult mice, STZ is administered to neonatal mice ory chaw, and they found emodin (1) treatment improved liv-
(STAM model) [64]. Generally, hepatic chemotoxins are con- er injury and weight normalization. In addition, blood lipid
tributable to weight reduction in mouse models. The corres- and hepatic triglyceride in emodin group markedly reduced,
ponding alterations which are similar to CDAA and MCD which may contribute to the increased expression of PPAR-
diet lead to the limitations of their utilities for demonstrating γ [69]. It has also been reported that myricetin (2) and plumba-
etiology and nature history of NASH, thus the primary ap- gin (3) lowered hepatic lipid levels possibly by altering
plications are on initial pathogenic factors. PPAR signaling [70-71].
Combined models AMPK is a ubiquitous heterotrimeric serine/threonine
The limitations of existing models led us to develop kinase that acts as a vital cellular energy sensor and a pivotal
mouse models of NASH that meets many criteria for a relev- regulator of cellular metabolism. Therefore, some natural
ant NASH model outlined above. The combination of mul- compounds have been found to regulate AMPK activity and
tiple interventions could make up for respective disadvant- functions as lipid modulator in NASH models. Guo and his
ages and more faithfully mimic the human NASH. For in- colleagues reported that baicalin (4) impeded NASH progres-
stance, a model administered a Western diet (WD) with sion in HFD-fed rats through promoting AMPK and ACC
weekly CCl4 also appears to closely resemble human phosphorylation [72]. Berbamine (5), a natural bisbenzyl-
NASH [65]. As mentioned above, ob/ob and db/db mice fail to isoquinoline alkaloid derived from Berberisamurensis, was
develop fibrosis without a second hit, so their combination reported to allay intracellular lipid accumulation. Sharma et
with MCD diet will supply the deficiency. al. found that berbamine (5) could attenuate lipid accumula-
In the streptozotocin high-fat diet model (STAM), tion via AMPK/mTOR/SREBP-1c axis [73]. In addition, Nect-
– 16 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
andrin B (6) and daphnetin (7) have been proved to particip- ferine (9) were reported to effectively decreased fatty acid,
ate in the activation of AMPK [74-75]. Theacrine (8) and nuci- further molecular investigations indicated their improvement
Table 3 Summary of natural compounds displaying anti-nonalcoholic steatohepatitis activity
Biological function
N° Compound Applied model Ref
towards NASH
(1) Emodin High caloric labortary chaw diet-fed rats [69]
(2) Myricetin HFD-fed mice [70]
(3) Plumbagin fructose feeding rats [71]
(4) Baicalin HFD-fed rats [72]
(5) Berbamine Oleic acid (OA) stimulated HepG2 cells [73]
(6) Nectandrin B Insulin plus high glucose-induced HepG2 cells [74]
(7) Daphnetin oleic acid (OA) induced HepG2 cells [75]
(8) Theacrine HFD-fed mice (ApoE-/- and C57BL/6J), Oleate-treated HepG2 and L-02 cells [76]
(9) Nuciferine Oleic acid (OA) stimulated HepG2 cells [77]
(10) Luteolin palmitate-stimulated HepG2 cells [78]
(11) Oxymatrine High-fat and high-fructose diet (HFDHFr)-fed rats [79]
Regulating metabolism (12) Tomatidine palmitate-stimulated HepG2 cells [80]
(13) Oleanolic acid High-fat high-cholesterol (HFHC) diet-fed rats [81]
Nordihydroguaiaretic
(14) American Lifestyle-Induced Obesity Syndrome (ALIOS) diet-induced mice [82]
acid
(15) Schizandrin A High-fat high-cholesterol (HFHC) diet-fed mice [83]
(16) Dioscin HFD-fed mice, ob/ob mice [84]
(17) Acetylshikonin db/db mice [85]
(18) Betulinic acid HFD-fed mice [86, 87]
(19) Cycloastragenol HFD-fed mice, MCD-fed mice [88]
HFD-fed and diethylnitrosamine (DEN) induced,HFD-fed rats, palmitate-
(20) Tiliamosine [89]
oleate induced HepG2 cells
(21) Ginsenoside Rb1 db/db mice [90]
(22) Sophocarpine Oleic acid (OA) stimulated primary hepatocytes from rats [91]
(23) Silybinin MCD-fed db/db mice [94]
Cyanidin 3-O-β-D-
(24) db/db mice [95]
glucoside
(25) Resveratrol HFD-fed mice, rats; fructose-fed rats [97, 150, 151]
(26) Quercetin Bile duct ligation (BDL), CCl4 administration [152, 153]
(27) Carnosic acid HFD-fed mice [99]
(28) Scutellarin HFD-fed mice, oleic acid (OA) stimulated HepG2 cells [100]
Regulating inflammation HFD,MCD-fed mice, palmitic acid-stimulated L02 hepatocytes; high-
(29) Matrine [101]
fructose diet-fed rats
(30) Geniposide Oleic acid (OA) plus palmitic acid (PA) stimulated HepG2 cells [103]
(31) Salvianolic acid A HFD-fed rats, palmitic acid (PA) treated HepG2 cells [104]
(32) Digoxin HFD-fed mice [105]
(33) Koumine HFD-fed rats [106]
(34) Schisandrin B LPS-activated RAW264.7 macrophages [107]
(35) Kukoamine B High-fat diet/high-fructose (HFDFr)-fed rats [108]
– 17 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
Continued
Biological function
N° Compound Applied model Ref
towards NASH
(36) Geraniol MCD-fed rats [109]
(37) Betaine MCD-fed mice [110]
(38) Genistein MCD fed db/db mice; HFD-fed rats [112, 154]
MCD-fed mice; LPS plus oleic acid (OA) stimulated HepG2 cells,
(39) Naringenin [113]
primary hepatocytes and Kupffer cells
(40) Scoparone MCD-fed mice [114]
(41) Withaferin A HFD-fed rats [117]
Epigallocatechin-3-
(42) HFD-fed rats; HFD and CCl4-induced SHRSP-ZF rats [122, 123]
gallate(EGCG)
(43) Isorhamnetin Combination of diet and chemical inducers (HFD+CCl4) [124]
(44) Astragaloside IV CCl4 induced mouse model [125]
−/−
(45) 1,8-Cineole Pten mice [128]
(46) Glycyrrhizic acid MCD-fed mice [129]
Regulating fibrosis
(47) Xanthohumol Paigen diet-fed mice and rats [130]
(48) Curcumin MCD-fed mice [132, 134]
(49) Thymoquinone High-fat high-cholesterol (HFHC) diet-fed rats [136]
(50) Isochlorogenic acid B MCD-fed mice [137]
(51) Conophylline Streptozotocin-induced rats; MCD-fed db/db mice [139, 155]
Regulating intestinal (52) Berberine HFD-fed mice, rats [143, 144]
microbiota (53) Salidroside HFD-fed mice [149]
PPAR ACC Fibrosis
Metabolism FGF21 AMPK
FXR Isorhamnetin
Glycyrrhizic acid
Curcumin
···
Emodin
Baicalin
Oleanolic acid
Collagen
deposition
Gut-liver
crosstalk
Proinflammatory cytokines
Chemokines
Intestinal microbiota ASK1 CCR2/5
Inflammation
TLR4 NLRP3
Silybinin
Berberine
LPS Scutellarin
Salidroside Matrine
··· ···
Fig. 1 The natural compounds and the relevant events in NASH
– 18 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
in energy expenditure [76-77]. liver weight. The beneficial effect of ginsenoside Rb1 (21)
Constituents regulating metabolism are often character- was primarily attributed to the reduction of ectopic lipid ac-
ized by the interference in de novo lipogenesis or fatty acid cumulation and lipolysis in adipocytes, which may result
oxidation. Luteolin (10), tomatidine (11), oxymatrine (12) from the increasing level of adiponectin [90]. The elevated
and oleanolic acid (13) were reported to regulate glucose leptin and adiponectin were also observed in OA-induced
homeostasis and lipid synthesis via reducing SREBP-1c, primary hepatocytes from rats after sophocarpine (22) treat-
ACC and FAS expression [78-81]. Nordihydroguaiaretic acid ment [91].
(14) or schizandrin A (15) supplement alleviated obesity par- Regulating inflammation
tially contributing to the elevated fatty acid oxidation [82-83]. In Silymarin, an extract from a milk thistle plant, has been
common leptin-deficient murine models, dioscin (16) or acet- extensively documented for prevention from liver injur-
ylshikonin (17) treatment suppressed total triglyceride syn- ies [92-93]. Silybinin (23), the major bioactive component of
thesis and attenuated liver injuries in ob/ob and db/db mice silymarin, was capable of attenuating hepatic steatosis, hep-
respectively [84-85]. Betulinic acid (18), tiliamosine (19) and atocyte ballooning and lobular inflammation in db/db mice
cycloastragenol (20) can activate FXR to improve ER stress, fed the MCD diet [94]. Cyanidin 3-O-β-D-glucoside (24) was
cholesterol metabolism, and simultaneously repress metabol- also reported to abate hepatic steatosis, neutrophil infiltration
ic disorders [86-89]. Ginsenoside Rb1 (21), the major bioactive in db/db mice [95].
component of ginseng, exerts anti-diabetic and anti-insulin Resveratrol (25) and quercetin (26) have been elucidated
resistance effects. Ginsenoside Rb1 (21) treatment could in- to have anti-inflammation activity for NASH based on simil-
crease insulin sensitivity, and reduce hepatic lipid content and ar mechanisms. Resveratrol (25) is established for the antiox-
OH
OH
O
OH O OH HO OCH3H3CO
CH3 GluO
O OH O H H
O HCN OCH3 N CH
HO 3 3
H 3C OCH3 OH O OCH3
OH O OH O O
O OH OH O
OH
Emodin (1) Myricetin (2) Plumbagin (3) Baicalin (4) Berbamine (5)
O CH3 H3CO OH
OCH3 H3C N N
H3CO O O H3CO N CHHO O OH
O N N
3
HO O H
HO OH
OH CH3 CH3 OH O
Nectandrin B (6) Daphnetin (7) Theacrine (8) Nuciferine (9) Luteolin (10)
O H3CO
N
N H H3CO
O OH H3CO
H COOH
OH H3CO OH
N H H3CO
HO HO HO
O H H H3CO
OH
Oxymatrine (11) Tomatidine (12) Oleanolic acid (13) Nordihydroguaiaretic acid (14) Schizandrin A (15)
O
O
OH O OH
OH O H COOH O
RO
O H H
O H H
R = HO O HO O HO H3CO O
OH O OH H
HO HO O HO
HO HO
OH
Dioscin (16) Acetylshikonin (17) Betulinic acid (18) Cycloastragenenol (19)
R1O
OCH3 OH
OCH3 O
O
N NH H
H3C H O H N
R1 = −Glc (6-1) Glc H
R 2O
OCH3 HO R2 = −Glc (2-1) Glc H H
N
Tiliamosine (20) Ginsenoside Rb1 (21) Sophocarpine (22)
Fig. 2 The natural compounds regulating metabolism against NASH
– 19 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
idative, anti-inflammatory, anti-cancer, anti-obesity, and anti- progression and outcomes of patients with NASH [119]. Hepat-
aging properties [96]. Resveratrol (25) treatment suppressed ic construction is inevitably disrupted when excessive fibro-
hepatic steatosis, insulin resistance, and inflammation in HFD- genesis occurs, and fibrosis ordinarily indicates a higher like-
induced mouse models of NASH [66, 97]. Mechanically, res- lihood of progression to cirrhosis and other end-stage liver
veratrol (25) upregulates autophagy and Nrf2-mediated anti- diseases in NASH [120].
oxidant defence, downregulates NF-κB-mediated inflammat- Concerns were raised on the protective role of epigalloc-
ory response in hepatocytes and adipocytes [98]. In HFD-in- atechin-3-gallate (EGCG) (42) against NASH, which is one
duced mice and rats, carnosic acid (27), scutellarin (28) and of the major green tea catechins [121]. Apart from that EGCG
matrine (29) treatment morphologically alleviated inflam- has been previously reported for counteracting the activity of
matory histology involving in modulation of NF-κB path- TGF/SMAD, PI3K/Akt/FoxO1 and NF-κB cascades [122], it
ways [99-101]. Quercetin (26), a flavonoid typically exists in was found by Kochi et al. that EGCG (42) administration im-
fruits including broccoli, onions and leafy green vegetab- peded the development of liver fibrosis in a SHRSP-ZF rat
les [102]. In HFD-induced rodent NASH models, quercetin (26) model induced by HFD and CCl4 [123]. And isorhamnetin (43)
treatment improved hepatic steatosis, reduced inflammatory treatment showed protective effect on fibrosis through redu-
cell infiltration and portal fibrosis. Except for the mechan- cing fibrogenic markers expression in mice induced by com-
isms overlapped with resveratrol (25), quercetin (26) also binations of HFD diet and CCl4 [124]. Astragaloside, the major
curbs the generation of CYP2E1-mediated reactive oxygen active components of Radix Astragali, significantly amelior-
species (ROS), which is crucial in the pathogenesis of ated liver fibrosis via inhibition of TGFβ1/Smad pathway in
NASH [98]. Oxidative stress determined by ROS production CCl4 induced mouse model [125]. And astragaloside IV (44)
together with the SOD level was markly reduced after treat- has been proved by much evidence for its various pharmaco-
ment with geniposide (30) and salvianolic acid A (31) in logical activities including antioxidant stress, anti-inflammat-
palmitic acid (PA)-induced HepG2 cells [103-104]. NACHT, ory, anticancer and antifibrosis [126-127]. Administration with
LRR, and PYD domains-containing protein 3 (NLRP3) in- 1,8-Cineole (45) in PTEN-/- mice improved fibrosis stage and
flammasome activation tightly relevant to ROS production reduced Collagen Ⅰ expression [128].
was inhibited by digoxin (32) in HFD-fed mice [105]. The activation of HSCs, which is the central mediator of
Natural compounds have also shown to exert immun- liver fibrogenesis, was suppressed by interventions with gly-
omodulatory properties by inhibition of proinflammatory cy- cyrrhizic acid (46) and xanthohumol (47). In MCD-diet fed
tokines release through altering percentages of immune cells mice, glycyrrhizic acid (46) also exerted multiple hepatopro-
in the liver. Koumine (33) was documented to enhance the tective effects including inflammation amelioration and lipid
proportion of hepatic CD4+/CD8+ T cells, Treg cells in HFD- metabolism regulation apart from limiting pericellular
fed rats, indicating its regulating activity in immune sys- fibrosis and collagen deposition [129]. Xanthohumol (47) is a
tem [106]. In NASH murine models, schisandrin B (34) or promising compound for treating fibrosis in NASH for indu-
kukoamine B (35) treatment downregulated TNF-α, IL-1β cing activated HSC apoptosis in vitro in a dose dependent
and IL-6 levels [107-108]. Geraniol (36) or betaine (37) supple- manner without impairing viability of human primary hepato-
ment showed anti-inflammantory function through ameliorat- cytes at high doses [130].
ing mitochondrial dysfunction in rats and mice fed with Curcumin (48) is a polyphenol derived from the herbal
MCD [109-110]. Genistein (38) is a phytoestrogen mainly found remedy and spice turmeric [131], emerging as a hepatoprotect-
in soy and functions as antioxidant [111]. Genistein (38) treat- ive compound. Previous studies have proved the protective
ment alleviated liver inflammation and fibrosis via decline in effects of curcumin (48) toward several types of chemically
TNF-α and IL-1β in NASH mice induced by MCD diet [112]. induced hepatotoxicity, including CCl4 and alcoholic induc-
Similarily, naringenin (39) and scoparone (40) were dis- ed liver disease [132-133]. In a mouse model established by feed-
covered to markly inhibit hepatic inflammation in MCD ing MCD diet, curcumin (48) treatment significantly reduced
mice [113-114]. α-smooth muscle actin and restricted the fibrosis stage [134].
In preobese mice induced by HFD diet, evident metabol- With regard to the integrated prevention against NASH, its
ic improvements in hepatic insulin sensitivity, glucose toler- insulin-sensitizing function depends on decreasing hepatic
ance were observed after intervention with withaferin A (41) PTP1B expression and activity [135]. Thymoquinone (49) has
(WA), which has been reported to alleviate many metabolic been reported by Awad et al. for the potential of improving
disorders [115-116]. Abu Bakar and his colleagues found that hepatic steatosis, inflammatory, apoptotic status and fibrosis
WA (41) improved hepatic oxidative functions via augmen- in high-fat high-cholesterol (HFHC) diet-feeding rats [136]. Be-
ted antioxidant enzyme activities [117]. Nautral constituents ex- sides, in a mouse model induced by MCD-diet, isochlorogen-
hibiting anti-inflammatory potential are shown in Fig. 3. ic acid B (50) exhibited comprehensive anti-fibrosis actions,
Regulating fibrosis which encompass minimal fibrosis state, downregulating
Liver fibrosis is characterized by activation of HSCs and genes involved in fibrosis, suppressing multiple profibrogen-
excessive accumulation of ECM as aspects of pathology [118]. ic factors [137]. Nakade et al. and his colleagues investigated
Liver fibrosis is the primary determinant of clinical disease the effect of conophylline (51) (CnP), a vinca alkaloid isol-
– 20 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
OH OH
HOH2C O OH OH
OH
H3CO O O OH HO O HO HO O
HO HO OH
OGlc OH
O OH OH OH O
Silybinin (23) Cyanidin 3-O-β-D-glucoside (24) Resveratrol (25) Quercetin (26)
OH
OH O OCH3 OH
OH O H
HO H
HOOC GlcAO O N
H O HO COOH
HO O
H H HOH2C H OGlc HO OH
OH O N O
OH
Carnosic acid (27) Scutellarin (28) Matrine (29) Geniposide (30) Salvianolic acid A (31)
O
O
O
OH O OH
N
H H3CO OH
H OH H3CO O O
CH3 O H3CO
H N O HO NH NH N NH2
O
H3CO HO
HO
OH 3
Digoxin (32) Koumine (33) Schisandrin B (34) Kukoamine B (35)
OH
HO HO O H3CO
N HO O
CH2OH
O H3CO O O
H3CO O OH OH O
Geraniol (36) Betaine (37) Genistein (38) Naringenin (39) Scoparone (40)
H CH2OH
OH HO
H O
H H
O
OH
Withaferin A (41)
Fig. 3 The natural compounds regulating inflammation anti-NASH
ated from the tropical plant Ervatamia microphylla [138], in for bacterial diarrhea [142]. In HFD-fed mice, berberine (52)
MCD diet-fed db/db mice with a specific focus on steatosis, has been reported to regulate the relative abundance of both
inflammation, and fibrosis. CnP (51) was shown to inhibit Firmicutes and Bacteroidetes in the gut [143]. Subsequently,
steatohepatitis, which may due to lowered levels of hepatic Zhang et al. found that berberine (52) treatment could effect-
TGF-β and PPARα, thus leading to the attenuation of hepatic ively curbed the progression of NASH in HFD-fed rats via al-
steatosis, inflammation and fibrosis in mice [139]. ternating the gut microbiota construction [144]. Salidroside (53)
Regulating intestinal microbiota is the main active component of Roseroot, which has been es-
Despite the detailed mechanisms of microbiota dysbiosis tablished for its glycolipid metabolism-regulating and anti-in-
in the intestine on the pathogenesis of NASH are obscure to flammation properties [145-147]. Salidroside (53) can attenuate
date, the intestinal microecology might hinge on regulating liver lipid accumulation, inflammatory responses, and insulin
energy balance and metabolism to promote NASH. It has resistance in rats with NAFLD [148]. In murine NASH models
been reported that HFD- or fructose-induced dysbiosis may induced by HFD, treatment with salidroside (53) signific-
enhance energy harvest from ingested food and, together with antly ameliorated HFD-induced intestinal bacteria, and bile
inflammation, promote energy storage and insulin resistan- acid disorder, thus proving the potential role of salidroside
ce [140]. Dysbiosis can contribute to deterioration of the intest- (53) in NASH treatment via the gut microbiota-bile acid-FXR
inal epithelial barrier, resulting in translocation of inflamma- axis [149]. In addition, resveratrol (25) was reported to main-
tion-provoking bacterial products and metabolites (and even tain gut barrier integrity and restrict inflammation in intest-
intact bacteria) that reach the liver via the portal circulation to ines. Resveratrol (25) treatment led to metabolic endotox-
further enhance NAFLD and NASH progression [141]. emia reduction and gut microbial distribution alterations in
Berberine (52), distributed majorly in the Chinese medi- SD rats fed with HFD [150]. Natural compounds which can
cine Coptis chinensis, is originally known for its clinical use regulate fibrosis and intestinal microbiota are shown in Fig. 4.
– 21 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
OH OH
OH
OCH3 O
HO O OH OH
O HO O OH O
OH O OH
OH XylO
OH OH O
OGlc
OH
Eplgallocatechin-3-gallate (42) Isorhamnetin (43) Astragaloside IV (44) 1, 8-Chineole (45)
COOH
H
O
H O O O
RO HO OH OH H CO OCH3
3
R = GlcA (2-1) GlcA HO OH
H3CO O O
Glycyrrhizic acid (46) Xanthohumol (47) Curcumin (48) Thymoquinone (49)
O
OCH3
O
H3CO OH
HO O OH O O
HO O O HN N O N
OH
H3COOC H N H OCH3 GlcO
OCH3
O NH COOCH3
HO HO
OH
Isochlorogenic acid B (50) Conophylline (51) Berberine (52) Salidroside (53)
Fig. 4 Regulating fibrosis (42−51) and intestinal microbiota (52, 53) anti-NASH
Discussion strong potential for natural products in the treatment for
NASH, but their appeal for clinical use warrants further in-
NASH is a worldwide chronic liver disease which brings
vestigation.
huge economic and health burden. The pathological initi-
ation of NASH is complicated and no drugs have been ap- Abbreviations
proved. There is a large body of events such as glycolipid
NASH, nonalcoholic steatohepatitis; NAFLD, nonalco-
metabolism dysregulation, ER stress, mitochondrial dysfunc-
holic fatty liver disease; TCMs, traditional Chinese medi-
tion, impaired immune systems, cytokine secretion, and the cines; ER, endoplasmic reticulum; ASK-1, apoptosis signal-
microbiota dysbiosis, correlating positively with NASH de- ing kinase-1; NF-κB, nuclear factor-kappa B; MAP3K, mito-
velopment. The progression to other end-stage liver diseases gen-activated protein kinase kinase kinase; JNK, Jun N-ter-
render it more urgent to develop available agents for clinical minal kinase; TLR4, Toll-like receptor 4; MyD88, myeloid
application. differentiation primary response gene 88; NLRP3, NOD-like
The applications of existing agents and compounds un- receptor protein 3; IL-1β, interleukin-1β; TNFα, tumor nec-
der evaluation are limited for the lack of effectiveness or vari- rosis factor α; LPS, lipopolysaccharide; CCR, Chemokine re-
ous side-effects. Accumulating evidences from experimental ceptors; GPCRs, G-protein coupled receptors; CCL2,
studies indicates that a number of natural products from chemokine ligand 2; FDA, Food and Drug administration;
TCMs can attenuate NASH through underlying mechanisms. MetS, metabolic syndrome; FGF, fibroblast growth factors;
Over the past centuries, TCMs have exerted preventative and GLUT1, glucose transporter type 1; SREBP1c, steroyl-CoA
curative applications on NAFLD. Quantities of TCMs are un- response element binding protein-1c; ACC, acetyl-CoA
der clinical evaluation despite the obscure of primary active carboxylase; FXR, farnesoid X receptor; OCA, obeticholic
components. It’s essential to search potential natural products acid; MCD, methionine and choline deficient diet; CDAA,
in the treatment for NASH, but to date the results of clinical choline-deficient l-amino acid defined diet; DIO, diet-in-
studies are limited. duced obesity; HFD, high-fat diet; MUP, major urinary pro-
In summary, the review demonstrates the pathological tein; uPA, urokinase- type plasminogen activator; PTEN,
features and the interrelated processes participating in NASH phosphatase and tensin homolog; AOX, acyl-coenzyme A ox-
pathogenesis, the hot targets that have been frequently stud- idase; MAT1A, methionine adenosyltransferase 1A; RNA-
ied and the animal models aiming at different objectives were seq, RNA-sequencing; CCl4, carbon tetrachloride; TAA,
also stated. Natural constituents that have anti-nonalcoholic thioacetamide; STZ, streptozotocin; WD, western diet; DIA-
steatohepatitis abilities are conducted, which suggests the MOND, Diet Induced Animal Model of Non-alcoholic Fatty
– 22 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
Liver Disease; ROS, reactive oxygen species; NLRP3, ease [J]. Adv Clin Chem, 2013, 59: 155-201.
NACHT, LRR, and PYD domains-containing protein 3; WA, [19] Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like re-
ceptor-4 signaling and Kupffer cells play pivotal roles in the
withaferin A; OA, oleic acid; HFDHFr, high-fat and high-
pathogenesis of non-alcoholic steatohepatitis [J]. J Hepatol,
fructose diet; HFHC, high-fat high-cholesterol diet; ALIOS, 2007, 47(4): 571-479.
American Lifestyle-Induced Obesity Syndrome; DEN, di- [20] Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflam-
ethylnitrosamine; BDL, bile duct ligation; PA, palmitic acid; masome blockade reduces liver inflammation and fibrosis in
HFDFr, high-fat diet/high-fructose; EGCG, epigallocatechin- experimental NASH in mice [J]. J Hepatol, 2017, 66(5): 1037-
1046.
3-gallate
[21] Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3
References inflammasome instigates obesity-induced inflammation and
insulin resistance [J]. Nat Med, 2011, 17: 179-188.
[1] Younossi ZM, Koenig AB, Abdelatif D, et al. Global epi- [22] Marra F, Tacke F. Roles for chemokines in liver disease [J].
demiology of nonalcoholic fatty liver disease: Meta-analytic Gastroenterology, 2014, 147(3): 577.
assessment of prevalence, incidence, and outcomes [J]. Hep- [23] Friedman SL, Neuschwander-Tetri BA, Rinella M, et al.
atology, 2016, 64(4): 73-84. Mechanisms of NAFLD development and therapeutic
[2] Zhou F, Zhou JH, Wang WX, et al. Unexpected rapid in- strategies [J]. Nat Med, 2018, 24: 908-922.
crease in the burden of NAFLD in China from 2008 to 2018: [24] Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to
A systematic review and meta-analysis [J]. Hepatology, 2019, macrophage infiltration into adipose tissue, insulin resistance,
70(4): 1119-1133. and hepatic steatosis in obesity [J]. J Clin Invest, 2006,
[3] Angulo P, Lindor K. Non-alcoholic fatty liver disease [J]. J 116(6): 1494-1505.
Gastroenterol Hepatol, 2002, 17(S1): S186-S190. [25] Pérez-Martínez L, Pérez-Matute P, Aguilera-Lizarraga J, et al.
[4] Angulo P. Nonalcoholic fatty liver disease [J]. N Engl J Med, Maraviroc, a CCR5 antagonist, ameliorates the development
2002, 346: 1221-1231. of hepatic steatosis in a mouse model of non-alcoholic fatty
[5] Ibrahim SH, Hirsova P, Malhi H, et al. Animal models of non- liver disease (NAFLD) [J]. J Antimicrob Chemother, 2014,
alcoholic steatohepatitis: Eat, delete, and inflame [J]. Dig Dis 69(7): 1903-1910.
Sci, 2016, 61: 1325-1336. [26] Berres ML, Koenen RR, Rueland A, et al. Antagonism of the
[6] Vilar-Gomez E, Chalasani N. Non-invasive assessment of non- chemokine Ccl5 ameliorates experimental liver fibrosis in
alcoholic fatty liver disease: Clinical prediction rules and mice [J]. J Clin Invest, 2010, 120(11): 4129-4140.
blood-based biomarkers [J]. J Hepatol, 2018, 68(2): 305-315. [27] Lefebvre E, Hashiguchi T, Jenkins H, et al. Anti-fibrotic and
[7] Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for Anti-inflammatory Activity of the Dual CCR5 and CCR2 Ant-
NASH: Current and emerging [J]. J Hepatol, 2018, 68(2): 362- agonist Cenicriviroc in a Mouse Model of NASH [C]. 15th In-
375. ternational Workshop on Co-morbidities and Adverse Drug
[8] Zhou JH, Zhou F, Wang WX, et al. Epidemiological features Reaction, 2013.
of NAFLD from 1999 to 2018 in China [J]. Hepatology, 2020, [28] Huang PL. A comprehensive definition for metabolic syn-
71(5): 1851-1864. drome [J]. Dis Model Mech, 2009, 2: 231-237.
[9] Yao H, Qiao YJ, Zhao YL, et al. Herbal medicines and nonal- [29] Babaknejad N, Nayeri H, Hemmati R, et al. An overview of
coholic fatty liver disease [J]. World J Gastroenterol, 2016, FGF19 and FGF21: The therapeutic role in the treatment of
22(30): 6890-6905. the metabolic disorders and obesity [J]. Horm Metab Res,
[10] Das K, Kar P. Non-alcoholic steatohepatitis [J]. J Assoc Phys- 2018, 50: 441-452.
icians India, 2005, 53: 195-199. [30] Lee DV, Li D, Yan Q, et al. Fibroblast growth factor 21 im-
[11] Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, proves insulin sensitivity and synergizes with insulin in hu-
hepatic insulin resistance, and type 2 diabetes [J]. Hepatology, man adipose stem cell-derived (hASC) adipocytes [J]. PLoS
2014, 59: 713-723. ONE, 2014, 9: e111767.
[12] Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutri- [31] Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 re-
tion, and non-alcoholic fatty liver disease [J]. Clin Biochem, verses hepatic steatosis, increases energy expenditure, and im-
2015, 48(13-14): 923-930. proves insulin sensitivity in diet-induced obese mice [J]. Dia-
[13] Bunkoczi G, Salah E, Filippakopoulos P, et al. Structural and betes, 2009, 58(1): 250-259.
functional characterization of the human protein kinase [32] Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for
ASK1 [J]. Structure, 2007, 15(10): 1215-1226. metabolic syndrome [J]. J Lipid Res, 2009, 50: S138-S143.
[14] Romero F, Jones C, Xu YZ, et al. The race to bash NASH: [33] Saggerson D. Malonyl-CoA, a key signaling molecule in
Emerging targets and drug development in a complex liver mammalian cells [J]. Annu Rev Nutr, 2008, 28: 253-272.
disease [J]. J Med Chem, 2020, 63(10): 5031-5073. [34] Ross TT, Crowley C, Kelly KL, et al. Acetyl-CoA
[15] Budas G, Karnik S, Jonnson T, et al. Reduction of liver ste- carboxylase inhibition improves multiple dimensions of
atosis and fibrosis with an Ask1 inhibitor in a murine model NASH pathogenesis in model systems [J]. Cell Mol Gastroen-
of Nash is accompanied by improvements in cholesterol, bile terol Hepatol, 2020, 10(4): 829-851.
acid and lipid metabolism [J]. J Hepatol, 2016, 64(2): S170. [35] Han CY. Update on FXR biology: Promising therapeutic tar-
[16] Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor get? [J]. Int J Mol Sci, 2018, 19(7): 2069.
selonsertib in patients with nonalcoholic steatohepatitis: A [36] Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essen-
randomized, phase 2 trial [J]. Hepatology, 2018, 67(2): 549- tial for normal glucose homeostasis [J]. J Clin Invest, 2006,
559. 116(4): 1102-1109.
[17] Guo J, Friedman SL. Toll-like receptor 4 signaling in liver in- [37] Zhang YQ, Castellani LW, Sinal CJ, et al. Peroxisome prolif-
jury and hepatic fibrogenesis [J]. Fibrogenesis Tissue Repair, erator-activated receptor-gamma coactivator 1alpha (PGC-1al-
2010, 3: 21. pha) regulates triglyceride metabolism by activation of the
[18] Petrasek J, Csak T, Szabo G. Toll-like receptors in liver dis- nuclear receptor FXR [J]. Genes Dev, 2004, 18: 157-169.
– 23 –LENG Ying-Rong, et al. / Chin J Nat Med, 2021, 19(1): 12-27
[38] Fiorucci S, Rizzo G, Antonelli E, et al. A farnesoid X receptor- Hepatol Res, 2006, 36(3): 217-228.
small heterodimer partner regulatory cascade modulates tis- [58] Nakagawa H, Umemura A, Taniguchi K, et al. ER stress co-
sue metalloproteinase inhibitor-1 and matrix metalloprotease operates with hypernutrition to trigger TNF-dependent spon-
expression in hepatic stellate cells and promotes resolution of taneous HCC development [J]. Cancer Cell, 2014, 26(3): 331-
liver fibrosis [J]. J Pharmacol Exp Ther, 2005, 314(2): 584- 343.
595. [59] Grompe M. Sandgren EP, Palmiter RD, Heckel JL, Daugh-
[39] Pellicciari R, Fiorucci S, Camaioni E, et al. 6α-ethyl-chen- erty CC, Brinster RL, Degen JL. Complete hepatic regenera-
odeoxycholic acid (6-ECDCA), a potent and selective FXR tion after somatic deletion of an albumin–plasminogen activ-
agonist endowed with anticholestatic activity [J]. J Med ator transgene [Cell 1991;66:245–256] [J]. J Hepatol, 2002,
Chem, 2002, 45(17): 3569-3572. 37(4): 422-424.
[40] Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety [60] Maurel M, Samali A, Chevet E. Endoplasmic reticulum stress:
of the farnesoid X receptor agonist obeticholic acid in pa- at the crossroads of inflammation and metabolism in hepato-
tients with type 2 diabetes and nonalcoholic fatty liver cellular carcinoma development [J]. Cancer Cell, 2014, 26:
disease [J]. Gastroenterology, 2013, 145(3): 574-582. 301-3.
[41] Jiang C, Xie C, Li F, Zhang L, et al. Intestinal farnesoid X re- [61] Teufel A, Itzel T, Erhart W, et al. Comparison of gene expres-
ceptor signaling promotes nonalcoholic fatty liver disease [J]. sion patterns between mouse models of nonalcoholic fatty liv-
J Clin Invest, 2015, 125(1): 386-402. er disease and liver tissues from patients [J]. Gastroentero-
[42] Xie C, Jiang C, Shi J, et al. An intestinal farnesoid X receptor- logy, 2016, 151(3): 513-525.
ceramide signaling axis modulates hepatic gluconeogenesis in [62] Constandinou C, Henderson N, Iredale JP. Modeling liver
mice [J]. Diabetes, 2017, 66(3): 613-626. fibrosis in rodents [J]. Methods Mol Med, 2005, 117: 237-250.
[43] Gonzalez FJ, Jiang C, Patterson AD. An intestinal microbiota- [63] Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin [J].
farnesoid X receptor axis modulates metabolic disease [J]. Mutat Res-Rev Mutat Res, 2002, 512(2-3): 121-134.
Gastroenterology, 2016, 151(5): 845-859. [64] Fujii M, Shibazaki Y, Wakamatsu K, et al. A murine model
[44] Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X re- for non-alcoholic steatohepatitis showing evidence of associ-
ceptor inhibition improves obesity-related metabolic dysfunc- ation between diabetes and hepatocellular carcinoma [J]. Med
tion [J]. Nat Commun, 2015, 6: 10166. Mol Morphol, 2013, 46: 141-152.
[45] Nakae D, Mizumoto Y, Andoh N, et al. Comparative changes [65] Tsuchida T, Lee YA, Fujiwara N, et al. Corrigendum to "A
in the liver of female Fischer-344 rats after short-term feeding simple diet- and chemical-induced murine NASH model with
of a semipurified or a semisynthetic L-amino acid-defined rapid progression of steatohepatitis, fibrosis and liver
choline-deficient diet [J]. Toxicol Pathol, 1995, 23(5): 583- cancer"[J Hepatol 69 (2018) 385-395] [J]. J Hepatol, 2018,
590. 69(4): 988.
[46] Hebbard L, George J. Animal models of nonalcoholic fatty [66] Radosavljević T, Mladenović D, Vucević D, et al. [The role of
liver disease [J]. Nat Rev Gastroenterol Hepatol, 2011, 8(1): oxidative/nitrosative stress in pathogenesis of paracetamol-in-
34-44. duced toxic hepatitis [J]. Med Pregl, 2010, 63(11-12): 827-
[47] Kodama Y, Kisseleva T, Iwaisako K, et al. c-Jun N-terminal 832.
kinase-1 from hematopoietic cells mediates progression from [67] Subramanian S, Goodspeed L, Wang S, et al. Dietary choles-
hepatic steatosis to steatohepatitis and fibrosis in mice [J]. terol exacerbates hepatic steatosis and inflammation in obese
Gastroenterology, 2009, 137(4): 1467-1477. LDL receptor-deficient mice [J]. J Lipid Res, 2011, 52: 1626-
[48] Deng QG, She H, Cheng JH, et al. Steatohepatitis induced by 1635.
intragastric overfeeding in mice [J]. Hepatology, 2005, 42(4): [68] Ka SO, Bang IH, Park BH. The protein kinase 2 inhibitor tet-
905-914. rabromobenzotriazole protects against renal ischemia reperfu-
[49] Ito M, Suzuki J, Tsujioka S, et al. Longitudinal analysis of sion injury [J]. Free Radical Biol Med, 2015, 86: S23.
murine steatohepatitis model induced by chronic exposure to [69] Dong H, Lu FE, Gao ZQ, X et al. Effects of emodin on treat-
high-fat diet [J]. Hepatol Res, 2007, 37(1): 50-57. ing murine nonalcoholic fatty liver induced by high caloric
[50] Matsumoto M, Hada N, Sakamaki Y, et al. An improved laboratory chaw [J]. World J Gastroenterol, 2005, 11(9): 1339-
mouse model that rapidly develops fibrosis in non-alcoholic 1344.
steatohepatitis [J]. Int J Exp Pathol, 2013, 94(2): 93-103. [70] Xia SF, Le GW, Wang P, et al. Regressive effect of myricetin
[51] Diehl AM. Lessons from animal models of NASH [J]. Hep- on hepatic steatosis in mice fed a high-fat diet [J]. Nutrients,
atol Res, 2005, 33(2): 138-144. 2016, 8(12): 799.
[52] Mayer J, Bates MW, Dickie MM. Hereditary diabetes in ge- [71] Pai SA, Munshi RP, Panchal FH, et al. Plumbagin reduces
netically obese mice [J]. Science, 1951, 113(2948): 746-747. obesity and nonalcoholic fatty liver disease induced by
[53] Takahashi Y, Soejima Y, Fukusato T. Animal models of non- fructose in rats through regulation of lipid metabolism, in-
alcoholic fatty liver disease/nonalcoholic steatohepatitis [J]. flammation and oxidative stress [J]. Biomed Pharmacother,
World J Gastroenterol, 2012, 18(19): 2300-2308. 2019, 111: 686-694.
[54] Poekes L, Legry V, Farrell G, et al. Role of ciliary dysfunc- [72] Guo HX, Liu DH, Ma Y, et al. Long-term baicalin administra-
tion in a new model of obesity and non-alcoholic steatohepat- tion ameliorates metabolic disorders and hepatic steatosis in
itis: the foz/foz mice [J]. Arch Public Health, 2014, 72: O7. rats given a high-fat diet [J]. Acta Pharmacol Sin, 2009, 30:
[55] Heydet D, Chen LX, Larter CZ, et al. A truncating mutation 1505-1512.
of Alms1 reduces the number of hypothalamic neuronal cilia [73] Sharma A, Anand SK, Singh N, et al. Berbamine induced
in obese mice [J]. Dev Neurobiol, 2013, 73: 1-13. AMPK activation regulates mTOR/SREBP-1c axis and
[56] Fan W, Boston BA, Kesterson RA, et al. Role of melano- Nrf2/ARE pathway to allay lipid accumulation and oxidative
cortinergic neurons in feeding and the agouti obesity syn- stress in steatotic HepG2 cells [J]. Eur J Pharmacol, 2020,
drome [J]. Nature, 1997, 385: 165-168. 882: 173244.
[57] Okumura K, Ikejima K, Kon K, et al. Exacerbation of dietary [74] Choi du G, Kim EK, Yang JW, So et al. Nectandrin B, a lig-
steatohepatitis and fibrosis in obese, diabetic KK-Ay mice [J]. nan isolated from nutmeg, inhibits liver X receptor-α-induced
– 24 –You can also read