Expression and Possible Significance of ACE2 in the Human Liver, Esophagus, Stomach, and Colon - Hindawi.com
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Hindawi
Evidence-Based Complementary and Alternative Medicine
Volume 2021, Article ID 6949902, 9 pages
https://doi.org/10.1155/2021/6949902
Research Article
Expression and Possible Significance of ACE2 in the Human Liver,
Esophagus, Stomach, and Colon
Yiwen Liu,1,2 Qing Wu,2 Dongmei Wan,3 Huiqin He,2 Hailian Lin,1 Kelang Wang,1
Genxiang Que,1 Yuanyuan Wang,1 Yongjun Chen,4 Xiaoqing Tang,5
Lingbo Wu ,6 and Xuefeng Yang 1,2
1
The Affifiliated Nanhua Hospital, Department of Gastroenterology, Hengyang Medical School, University of South China,
Hengyang 421001, Hunan, China
2
The Affifiliated Nanhua Hospital, Department of General Practice, Hengyang Medical School, University of South China,
Hengyang 421001, Hunan, China
3
The Affifiliated Nanhua Hospital, Department of Orthopaedic Traumatology, Hengyang Medical School,
University of South China, Hengyang 421001, Hunan, China
4
The Affiliated Nanhua Hospital, Department of Neurology, Hengyang Medical School, University of South China,
Hengyang 421001, Hunan, China
5
The First Affiliated Hospital, Institute of Clinical Medicine, Hengyang Medical School, University of South China,
Hengyang 421001, Hunan, China
6
The Affifiliated Nanhua Hospital, Department of Respiratory Medicine, Hengyang Medical School, University of South China,
Hengyang 421001, Hunan, China
Correspondence should be addressed to Lingbo Wu; 919905940@qq.com and Xuefeng Yang; yxf009988@sina.com
Received 4 July 2021; Accepted 16 August 2021; Published 25 August 2021
Academic Editor: Songwen Tan
Copyright © 2021 Yiwen Liu et al. This is an open access article distributed under the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Angiotensin-converting enzyme 2 (ACE2) has been identified as the key receptor of SARS coronavirus that plays a key role in the
pathogenesis of SARS. It is known that ACE2 mRNA can be expressed in most organs. However, the protein expression of ACE2 is
not clear yet. To explore the role of ACE2 as a precipitating factor in digestive organ damage in COVID-19, this study investigated
the expression of ACE2 protein in the human liver, esophagus, stomach, and colon. The result showed that ACE2 can be expressed
in the liver, esophagus, stomach, and colon, which suggests SARS-CoV-2 may enter the digestive system through ACE2 and cause
liver damage and gastrointestinal damage. It is hoped that the result of the study will provide a new strategy for the prevention and
treatment of digestive organ damage under COVID-19.
1. Introduction angiotensinogen in the blood into inactive angiotensin I
(Ang I). In the plasma and tissues, especially on the surface
Angiotensin-converting enzyme 2 (ACE2) is a kind of trans- of the vascular endothelium of the pulmonary circulation,
membrane protein. Its gene is situated in the X chromosome. there is ACE2 that can hydrolyze Ang I to 8-peptide an-
The protein which is coded by the gene is composed of 805 giotensin II (Ang II). Ang II plays a role in the regulation of
amino acids, including the N-terminal signal peptide sequence blood pressure, proinflammatory, and apoptosis promoting
in the pericellular membrane, the C-terminal sequence in the through binding to the related receptors. Second, studies
transmembrane domain, and the intracellular [1–3]. have revealed that ACE2 acts on the Kinin–Kallikrein system
ACE2 generally plays a key role in the human body (KKS) by degrading dearginine bradykinin, a bradykinin
through three ways. First, it participates in the regulation analogue. Dearginine bradykinin can bind to the B1 re-
process of the renin angiotensin system (RAS). The renin ceptors which are expressed in tissues. Therefore, the release
secreted by the periglomerular cell of the kidney can convert of inflammatory factors is promoted, which plays an2 Evidence-Based Complementary and Alternative Medicine
important role in the process of inflammation as increasing Jinqiao Biotechnology Co., Ltd. Powder antigen repair so-
the production of local nitric oxide causes hemangiectasis lution (citric acid method), PBS phosphate buffer (powder),
[4, 5]. Third, ACE2 is closely associated with the incidence of ready-to-use immunohistochemical hypersensitive Ultra-
severe acute respiratory syndrome (SARS) in 2003 [6, 7]. In SensitiveTM immunohistochemical kit reagent, DAB color
2003, the SARS epidemic which is caused by severe acute kit, and Super PAP Pen Super Immunohistochemical Oil
respiratory syndrome coronavirus (SARS-CoV) spread all Pen were purchased from the Fuzhou Maixin Biotechnology
over the world. Based on the fact that Vero E6 cells could be Co., Ltd.
infected and replicated by SARS-CoV [8], Li’s team deter-
mined that ACE2 could effectively bind the S1 domain of
SARS-CoV S protein by applying the coimmunoprecipitation. 2.2. Specimens. Liver samples were collected from patients
Therefore, it was proved that ACE2 is the needful receptor for with hepatic hemangioma. Esophageal mucosa, gastric
SARS-CoV infection because it promotes the entry of the mucosa, and colonic mucosa were obtained from tissues
virus through the membrane fusion mechanisms [9]. The biopsied for diagnostic purposes. All the specimens were
novel coronavirus pneumonia 2019 (COVID-19) broke out in diagnosed by pathological examination. The mucosal tissues
late 2019. Its pathogen is named as severe acute respiratory were identified to be liver tissue, esophageal mucosal tissue,
syndrome coronavirus (SARS-CoV-2) by the International gastric tissue, and colonic mucosal tissue, with normal
Committee on Virology. It has triggered a global epidemic. structure by the Department of Pathology of the Affiliated
Studies have found that SARS-CoV-2 and SARS-CoV share a Nanhua Hospital of USC. All the patients had not received
high degree of similarity. Based on the fact that both of them any kind of treatment, such as radiotherapy, chemotherapy,
belong to the genus β-coronavirus, there is 80% homology in and targeted therapy and had not been exposed to any
terms of genome sequence [10]. But the ability of SARS-CoV- biochemical toxicants. Research followed the tenets of the
2 to bind the S protein and ACE2 is much stronger than that Declaration of Helsinki. Study protocols were approved by
of SARS-CoV [11]. We hypothesized COVID-19 that is the Institutional Review Board at the Affiliated Nanhua
caused by SARS-CoV-2 had similar pathogenesis with SARS Hospital of University of South China, and study partici-
which is caused by SARS-CoV. pants underwent informed consent by the treating digestive
In the early stage of our study, we found that except the physician.
obvious damage on the respiratory organ, damage on other
organs also showed in the 48 COVID-19 patients in the 2.3. Immunohistochemistry. All specimens were fixed by
Hengyang area, including the digestive organs. Among the 48 10% paraformaldehyde solution for about 12–24 hours and
patients with COVID-19, 27.1% of the patients had poor ap- embedded by paraffin. Paraffin-embedded tissue sections of
petite, 8.3% of the patients had nausea and vomiting, and 14.7% 5 μm thick were placed in a 60°C thermostat for 1 hour and
of the patients had diarrhea. With the primary diseases of the hydrated through a series steps of xylene and ethanol baths
liver, such as viral hepatitis, excluded, ALT was increased in to water. Deparaffinized sections were boiled in antigen
22.9% of patients, AST was increased in 20.8% of patients, ALP retrieval solution (citric acid method) for 5 min and cooled
was increased in 5.4% of patients, and c-GT increased in 21.6% down for 20 min, and a total of 4 repairs were practiced. In
of patients [12]. The data are accordant to the published study slides were added one drop of peroxidase blocking solution
[13]. The clinical symptom analysis of Xie’s team shows that and then washed with hot tap water for 1 min and PBS twice
ALT was increased in 31.6% of patients, AST was increased in for 3 min each time. After cleaning PBS, slides were incu-
35.4% of patients, and bilirubin was increased in 5.1% of bated with anti-ACE2 antibody and left overnight in 4°C in
patients [14]. Why do COVID-19 patients suffer from digestive the refrigerator. After 3 washes with PBS, the sections were
system damage? Is the injury caused by SARS-CoV-2 virus incubated with the secondary biotinylated goat anti-rabbit
directly or by the systemic inflammatory response (SIRS)? We IgG for 20 min at room temperature and rinsed with PBS
rarely know the mechanism of the digestive system damage in solution 3 times, 3 minutes each time. Slides were covered
COVID-19 patients. But the knowledge about it is significant totally by the preprepared DAB chromogenic solution and
for clinical treatment guidance. placed in room temperature for color development. When
In this study, immunohistochemistry was applied to light yellow is observed, slides were rinsed with tap water to
detect the expression of ACE2 protein in the human liver, stop the chromogenic reaction. The sections were also
esophagus, stomach, and colon. The role of ACE2 in the counterstained with hematoxylin counterstain in the nu-
COVID-19 digestive system damage was explored in the cleus and mounted with the mounting medium.
study, with the help of relevant references. New strategies for
the prevention and treatment of the COVID-19 digestive
system organ damage may be provided. 2.4. Result Judgment of Immunohistochemistry. The ex-
pression level of ACE2 was interpreted according to the
2. Materials and Methods staining results that were observed by the microscope. If the
expression of staining is light yellow, brownish yellow, or
2.1. Reagents. Rabbit monoclonal antibody to ACE2 was tan, then ACE2 is positive. Five observation sections of the
purchased from the Abcam Company. BSA was bought from high magnification field (×400) were randomly observed to
the Shanghai Shenggong Biology Co., Ltd. Hematoxylin observe the staining. We clarified the staining intensity of the
staining solution was bought from the Beijing Zhongshan cytoplasm and cell membrane of the stained cells andEvidence-Based Complementary and Alternative Medicine 3
determined the proportion of stained cells in all tissue cells 4. Discussion
to level staining. The staining intensity was divided into 0
(no positive cells), 1 (weak staining intensity of light yellow), ACE2 is expressed in many human organs and tissues. Its
2 (moderate staining of brownish yellow), and 3 (strong expression in the lung generally is located in the type II
staining of brownish brown). The percentage of positive alveolar epithelial cells [15]. Its expression in the kidney is
staining was scored as 0 (negative), 1 (1–25%), 2 (26–50%), 3 mainly located in renal tubule cells, but the expression
(51–75%), and 4 (76–100%). The staining score was the cannot be found in the immune cells and epidermal cells of
product of staining intensity and percentage of positive the glomerulus [16]. Some studies have shown that ACE2 is
staining. The score of 0 was judged as (−), 1–4 as weakly expressed in vascular endothelial cells, arterial smooth
positive (+), 5–8 as positive (++), and 9–12 as strongly muscle cells, and cardiac cells [17]. Significant myocardial
positive (++++). Immunohistochemistry scores were injury, including myocardial fibrosis and myocardial hy-
expressed as mean ± standard deviation, the MD ± SD. pertrophy, is seen in ACE2 knockout mice [18, 19]. In brain
tissue, ACE2 is expressed in a low level with only a small
3. Result amount of ACE2 expressed in glial cells and neurons of the
brain [20].
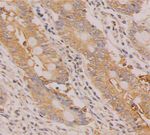
3.1. ACE2 Is Expressed in Human Liver Tissues. The positive In addition to the lung, kidney, and heart, ACE2 mRNA
ACE2 staining in liver tissues is observed brownish yellow. is also expressed in digestive organs, mainly in the gall-
The typical cytoplasmic staining indicates that ACE2 mainly bladder, bile duct, and stomach [21]. Another study shows
exists in the cytoplasm. No positive staining is found in the that the expression level of ACE2 mRNA in the digestive
cell membrane, while a little staining could be observed in system is higher than that in the lung [22]. The protein
the lumen of vascular endothelial cells and bile ducts. The expression of ACE2 in the digestive system has not been
magnification is 400× (Figures 1(a)–1(c)). According to the reported. In this study, the results of immunohistochemistry
immunohistochemical score, three liver tissue specimens showed that ACE2 protein was expressed in the liver,
were positive (++). stomach, esophagus, and colon. ACE2 expression in the liver
was mainly located in the cytoplasm of the liver, and no
3.2. ACE2 Is Expressed in Human Esophageal Mucosal Tissues. positive staining was observed on liver cell membrane. Slight
ACE2 is observed in the mucosal layer of esophageal tissue staining was observed in the vascular endothelial cells and
with brownish yellow staining. No positive staining was lumen of the bile ducts. The expression of ACE2 in
observed in other parts. It is mainly localized in the cyto- esophageal mucosa was mainly located in the epithelial
plasm. The magnification is 400× (Figures 1(d)–1(f )). cytoplasm, and no positive staining was observed in other
According to the immunohistochemical score, two cases in sites. ACE2 expression in gastric mucosa was mainly located
the group were positive (++) (Figures 1(d) and 1(e)) and one in the epithelial cytoplasm of gastric fundus gland of gastric
case was weakly positive (+) (Figure 1(f )). mucosa. ACE2 expression in colon tissue was mainly located
in the cytoplasm of colon mucosal epithelial cells, and
staining was observed in the brush border of villus. The
3.3. ACE2 Is Expressed in Human Gastric Mucosal Tissues.
ACE2 immunohistochemical staining strength score was in
If ACE2 is positive in human gastric mucosal tissues, the
the order of the liver, stomach, and esophagus, as the
staining is brownish yellow. All three specimens were ob-
weakest staining was observed in the esophageal mucosa.
tained from the fundic glands of the gastric mucosal epi-
According to the results, ACE2 was expressed in the liver,
thelium, and the positive expression is located in the
esophagus, stomach, and colon tissues.
cytoplasm. The magnification is 400× (Figures 1(g)–1(i)).
At present, novel coronavirus disease 2019 (COVID-19)
According to the immunohistochemical score, two cases in
that is caused by SARS-CoV-2 still threats human health all
this group were positive expression (++) (Figures 1(h) and
over the world. In addition to the lung damage, SARS-CoV-2
1(i)) and one showed mild positive (+) (Figure 1(g)).
also causes damage to organs of other systems, including the
digestive system. In the early stage of our study, we found
3.4. ACE2 Is Expressed in Human Colonic Mucosal Tissues. that among the 48 COVID-19 patients in the Hengyang area,
ACE2 was observed in colonic tissue with the brownish 27.1% of the patients developed poor appetite, 8.3% of the
yellow color. It was located in the cytoplasm. Staining is patients developed nausea and vomiting, and 14.7% of the
visible on the brush border of the villi. The magnification is patients developed diarrhea. Except for primary liver dis-
400× (Figures 1(j)–1(l)). Based on the immunohistochem- eases such as viral hepatitis, ALT was elevated in 22.9% of
ical score, two of the three specimens in the group showed patients, AST was increased in 20.8% of patients, ALP was
positive expression (++) (Figures 1(j) and 1(k)) and one increased in 5.4% of patients, and c-GT was increased in
showed litter positive (+) (Figure 1(l)). 21.6% of patients. It is significant to study the mechanism of
the digestive system damage in COVID-19 patients for
3.5. Grading of Immunohistochemistry. On the basis of Ta- guiding clinical treatment.
ble 1, it can be concluded that the liver tissue got the highest SARS-CoV-2 is a kind of single-stranded RNA virus. Its
score of ACE2 immunohistochemical staining intensity genome sequence contains 29,891 bases, and its virions are
score, followed by gastric tissue, colonic tissue, and spherical with a diameter of 60–140 nm. There are four kinds
esophageal mucosal tissue. of structural proteins in SARS-CoV-2 from the envelope toEvidence-Based Complementary and Alternative Medicine 7
(l)
Figure 1: (a)–(c) Expression and localization of ACE2 in human liver tissues. (d)–(f ) Expression and localization of ACE2 in human
esophageal mucosal tissues. (g)–(i) Expression and localization of ACE2 in human gastric mucosal tissues. (j)–(l) Expression and lo-
calization of ACE2 in human colonic mucosal tissues. Dewaxed liver tissue sections are treated with rabbit anti-ACE2 antibody for
immunohistochemistry. ACE2 expression was analyzed in human liver tissues ((a)–(c)), human esophageal mucosal tissue ((d)–(f )), human
gastric mucosal tissues ((g)–(i)), and human colonic mucosal tissues((j)–(l)). Boxed regions in the left of ((a)–(l)) are shown at higher
magnification in the right panel (400×).
Table 1: Immunohistochemical score of every tissue specimen.
Cytoplasm
Tissue Average score (MD ± SD)
Dyeing intensity Average positive area (%)
Liver M 86.7 8.00 ± 0.00
Esophageal mucosal M 55.3 5.30 ± 1.15
Gastric mucosal M 56.7 6.00 ± 2.00
Colonic mucosal M 56.7 6.00 ± 3.46
the virus core, the envelope protein (E protein), spinous for virus invasion. When S protein binds to ACE2 on the cell
process protein (S protein), membrane protein (M protein), surface, the former is activated by transmembrane protease
and nucleocapsid protein (N protein) [23, 24]. At present, serine 2 (TmRSS2), and thus, TMPRSS2S protein and ACE2
the pathogenesis of COVID-19 mainly includes ACE2- constitute protein complex and undergo conformational
mediated direct damage, cytokine storm, ischemia-hypoxia, changes. That divides S protein into S1 and S2 subunits,
and drug damage. Through the autopsy on COVID-19 which are attached to the host cell membrane and undergo
patients, Wang’s team found the apoptosis of the liver cell of membrane fusion, promoting SARS-CoV-2 to invade cells
COVID-19 patients was significant obvious, and there were for infection replication [28].
typical SARS-CoV-2 virus particles in the liver cells [25]. In the COVID-19 treatment studies, targeting ACE2
Another autopsy result showed that there were metamor- provides the possibility to prevent the invasion of SARS-
phosis, necrosis, and exfoliation in some epithelial cells in CoV-2, while maintaining RAS homeostasis may also help to
gastrointestinal mucosa [26]. These findings suggest that the reduce the damage to the digestive system. In their research,
digestive system damage in COVID-19 patients may be Zhang’s team established a colitis mouse model by using
caused by viral infection. Therefore, the high expression of dextran sulphate sodium salt to treat ACE2 gene knockout
ACE2 in the digestive system may suggest that the digestive mice and subsequently found that the level of AngII in the
tract may be the potential route of infection. damaged colon tissue increased. After using the human
The binding of ACE2 and S protein is the first step for the recombinant soluble ACE2 (HRSACE2), a lower level of
virus to invade cells. The S protein of SARS-CoV-2 envelope ANGII than before showed up [29]. Therefore, HRSACE2
is transmembrane glycoprotein that includes two subunits, can maintain the activity of ACE2 of host cell. Previous
S1 and S2. The receptor binding domain of S1 subunit studies have shown that HRSACE2 combined with SARS-
mediates the invasion through binding to the host cell re- CoV-2 can significantly inhibit virus infection [30]. ACEI
ceptors, while S2 subunit promotes the fusion of virus and and ARB have also been proved; they are effective in alle-
host cell membrane; therefore, it narrows the distance be- viating the progression of ARDS [31, 32]. But ACEI and ARB
tween them [27]. S protein can also be activated by a variety can increase the expression of ACE2, which may promote
of proteases (such as trypsin and elastase) to promote the the invasion of SARS-CoV-2. So that, the use of ACEI and
formation of syncytium on the cell surface, which is critical ARB in COVID-19 patients is still controversial. Studies8 Evidence-Based Complementary and Alternative Medicine
have shown that SARS-CoV-2 monoclonal antibody [4] W. B. Campbell, S. N. Brooks, and W. A. Pettinger, “An-
MAB4A8 can effectively neutralize SARS-CoV-2 and reduce giotensin II- and angiotensin III-induced aldosterone release
the binding of SARS-CoV-2 and ACE2 [33]. in vivo in the rat,” Science, vol. 184, no. 4140, pp. 994–996,
The results of this study showed that ACE2 was 1974.
expressed in the liver, esophagus, stomach, and colon tissue, [5] P. Hillmeister and P. B. Persson, “The Kallikrein-Kinin sys-
which suggests that SARS-CoV-2 may enter the digestive tem,” Acta Physiologica, vol. 206, no. 4, pp. 215–219, 2012.
[6] A. Shulla, T. Heald-Sargent, G. Subramanya, J. Zhao,
system through ACE2 and cause liver and gastrointestinal
S. Perlman, and T. Gallagher, “A transmembrane serine
damage.
protease is linked to the severe acute respiratory syndrome
coronavirus receptor and activates virus entry,” Journal of
Data Availability Virology, vol. 85, no. 2, pp. 873–882, 2011.
[7] S. Matsuyama, N. Nagata, K. Shirato, M. Kawase, M. Takeda,
The data used to support the findings of this study are and F. Taguchi, “Efficient activation of the severe acute re-
available from the corresponding author upon request. spiratory syndrome coronavirus spike protein by the trans-
membrane protease TMPRSS2,” Journal of Virology, vol. 84,
Disclosure no. 24, pp. 12658–12664, 2010.
[8] T. G. Ksiazek, D. Erdman, C. S. Goldsmith et al., “A novel
Yiwen Liu and Qing Wu are the co-first authors. coronavirus associated with severe acute respiratory syn-
drome,” New England Journal of Medicine, vol. 348, no. 20,
pp. 1953–1966, 2003.
Conflicts of Interest [9] W. Li, M. J. Moore, N. Vasilieva et al., “Angiotensin-con-
The authors declare that they have no conflicts of interest. verting enzyme 2 is a functional receptor for the SARS
coronavirus,” Nature, vol. 426, no. 6965, pp. 450–454, 2003.
[10] P. Zhou, X.-L. Yang, X.-G. Wang et al., “A pneumonia
Authors’ Contributions outbreak associated with a new coronavirus of probable bat
origin,” Nature, vol. 579, no. 7798, pp. 270–273, 2020.
Yiwen Liu and Xuefeng Yang were responsible for the de- [11] D. Wrapp, N. Wang, K. S. Corbett et al., “Cryo-EM structure
sign, implementation, and manuscript writing of the re- of the 2019-nCoV spike in the prefusion conformation,”
search. Qing Wu, Lingbo Wu, Dongmei Wan, and Huiqin Science, vol. 367, no. 6483, pp. 1260–1263, 2020.
He were in charge of specimen collection and data collation. [12] Z.-F. Zhong, J. Huang, X. Yang et al., “Epidemiological and
Hailian Lin, Kelang Wang, Genxiang Que, and Yuanyuan clinical characteristics of COVID-19 patients in Hengyang,
Wang were responsible for the literature review. Yongjun hunan Province, China,” World journal of clinical cases, vol. 8,
Chen and Xiaoqing Tang were responsible for revising the no. 12, pp. 2554–2565, 2020.
study. All authors participated in the analysis and inter- [13] C. Liu, Z. C. Jiang, C. X. Shao et al., “Preliminary study of the
pretation of the data and passed the final article. Yiwen Liu relationship between novel coronavirus pneumonia and liver
and Qing Wu made equal contributions to this work. function damage: a multicenter study,” Chinese journal of
hepatology, vol. 28, no. 2, pp. 107–111, 2020.
[14] H. Xie, J. Zhao, N. Lian, S. Lin, Q. Xie, and H. Zhuo, “Clinical
Acknowledgments characteristics of non-ICU hospitalized patients with coro-
navirus disease 2019 and liver injury: a retrospective study,”
This study was supported by Novel Coronavirus Pneumonia
Liver International, vol. 40, no. 6, pp. 1321–1326, 2020.
Emergency Project of University of South China (2020-15 [15] I. Hamming, W. Timens, M. Bulthuis, A. Lely, G. Navis, and
and 2020-25), Fund Project of Hengyang city for Prevention H. van Goor, “Tissue distribution of ACE2 protein, the
and Control of COVID-19 (2020hcjz6713), the Hengyang functional receptor for SARS coronavirus. A first step in
Science and Technology Plan Project-Basic Research Project understanding SARS pathogenesis,” The Journal of Pathology,
of Prevention and Treatment of the Novel Coronavirus vol. 203, no. 2, pp. 631–637, 2004.
Pneumonia (202010031577 and 202010031582), and Key [16] C. Fan, W. Lu, K. Li, Y. Ding, and J. Wang, “ACE2 expression
Research and Development Program of Hunan Province in kidney and testis may cause kidney and testis infection in
(2020SK3039 and 2020SK3040). COVID-19 patients,” Frontiers of Medicine, vol. 7, Article ID
563893, 2020.
[17] H. Xu, L. Zhong, J. Deng et al., “High expression of ACE2
References receptor of 2019-nCoV on the epithelial cells of oral mucosa,”
[1] J. Liu, X. Zheng, Q. Tong et al., “Overlapping and discrete International Journal of Oral Science, vol. 12, no. 1, p. 8, 2020.
aspects of the pathology and pathogenesis of the emerging [18] K. M. Baker, G. W. Booz, and D. E. Dostal, “Cardiac actions of
human pathogenic coronaviruses SARS-CoV, MERS-CoV, angiotensin II: role of an intracardiac renin-angiotensin
and 2019-nCoV,” Journal of Medical Virology, vol. 92, no. 5, system,” Annual Review of Physiology, vol. 54, no. 1,
pp. 491–494, 2020. pp. 227–241, 1992.
[2] N. Zhu, D. Zhang, W. Wang et al., “A novel coronavirus from [19] K. Yamamoto, M. Ohishi, T. Katsuya et al., “Deletion of
patients with pneumonia in China, 2019,” New England angiotensin-converting enzyme 2 accelerates pressure over-
Journal of Medicine, vol. 382, no. 8, pp. 727–733, 2020. load-induced cardiac dysfunction by increasing local angio-
[3] Y. Chen, Q. Liu, and D. Guo, “Emerging coronaviruses: ge- tensin II,” Hypertension, vol. 47, no. 4, pp. 718–726, 2006.
nome structure, replication, and pathogenesis,” Journal of [20] A. M. Baig, A. Khaleeq, U. Ali, and H. Syeda, “Evidence of the
Medical Virology, vol. 92, no. 4, pp. 418–423, 2020. COVID-19 virus targeting the CNS: tissue distribution, host-Evidence-Based Complementary and Alternative Medicine 9
virus interaction, and proposed neurotropic mechanisms,”
ACS Chemical Neuroscience, vol. 11, no. 7, pp. 995–998, 2020.
[21] L. Zou, F. Ruan, M. Huang et al., “SARS-CoV-2 viral load in
upper respiratory specimens of infected patients,” New En-
gland Journal of Medicine, vol. 382, no. 12, pp. 1177–1179,
2020.
[22] J. Xu, M. Chu, F. Zhong et al., “Digestive symptoms of
COVID-19 and expression of ACE2 in digestive tract organs,”
Cell death discovery, vol. 6, no. 1, p. 76, 2020.
[23] C. Huang, Y. Wang, X. Li et al., “Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China,” The
Lancet, vol. 395, no. 10223, pp. 497–506, 2020.
[24] J. Cui, F. Li, and Z.-L. Shi, “Origin and evolution of pathogenic
coronaviruses,” Nature Reviews Microbiology, vol. 17, no. 3,
pp. 181–192, 2019.
[25] Y. Wang, S. Liu, H. Liu et al., “SARS-CoV-2 infection of the
liver directly contributes to hepatic impairment in patients
with COVID-19,” Journal of Hepatology, vol. 73, no. 4,
pp. 807–816, 2020.
[26] X. H. Yao, T. Y. Li, Z. C. He et al., “A pathological report of
three COVID-19 cases by minimal invasive autopsies,” Chi-
nese journal of pathology, vol. 49, no. 5, pp. 411–417, 2020.
[27] H. R. Jonsdottir and R. Dijkman, “Coronaviruses and the
human airway: a universal system for virus-host interaction
studies,” Virology Journal, vol. 13, no. 1, p. 24, 2016.
[28] M. Hoffmann, H. Kleine-Weber, S. Schroeder et al., “SARS-
CoV-2 cell entry depends on ACE2 and TMPRSS2 and is
blocked by a clinically proven protease inhibitor,” Cell,
vol. 181, no. 2, pp. 271–280, e8, 2020.
[29] T. Hashimoto, T. Perlot, A. Rehman et al., “ACE2 links amino
acid malnutrition to microbial ecology and intestinal in-
flammation,” Nature, vol. 487, no. 7408, pp. 477–481, 2012.
[30] V. Monteil, H. Kwon, P. Prado et al., “Inhibition of SARS-
CoV-2 infections in engineered human tissues using clinical-
grade soluble human ACE2,” Cell, vol. 181, no. 4, pp. 905–913,
e7, 2020.
[31] R. M. Wösten-van Asperen, R. Lutter, P. A. Specht et al.,
“Acute respiratory distress syndrome leads to reduced ratio of
ACE/ACE2 activities and is prevented by angiotensin-(1-7) or
an angiotensin II receptor antagonist,” The Journal of Pa-
thology, vol. 225, no. 4, pp. 618–627, 2011.
[32] H. Liu and J. Zhao, “An experimental study of therapeutic
effect of ACEI on chemical-induced ARDS in rats,” Zhonghua
yu fang yi xue za zhi [Chinese journal of preventive medicine],
vol. 36, no. 2, pp. 93–96, 2002.
[33] X. Chi, R. Yan, J. Zhang et al., “A neutralizing human an-
tibody binds to the N-terminal domain of the Spike protein of
SARS-CoV-2,” Science, vol. 369, no. 6504, pp. 650–655, 2020.You can also read