Diselenide as a Dual Functional Mechanophore Capable of Stress Self-Reporting and Self-Strengthening in Polyurethane Elastomers
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
RESEARCH ARTICLE
Received: Feb. 14, 2022 | Accepted: Apr. 12, 2022 | Published: May 20, 2022
Diselenide as a Dual Functional
Mechanophore Capable of Stress
Self-Reporting and Self-Strengthening
in Polyurethane Elastomers
Xiaopei Li1, Fan Yang1, Yiran Li2, Cheng Liu3, Peng Zhao3, Yi Cao2, Huaping Xu3* & Yulan Chen4*
1
Department of Chemistry, Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin
300072, 2Department of Physics, Collaborative Innovation Center of Advanced Microstructures, National Laboratory
of Solid State Microstructure, Nanjing University, Nanjing, Jiangsu 210093, 3Department of Chemistry, Key Laboratory
of Organic Optoelectronics and Molecular Engineering, Tsinghua University, Beijing 100084, 4College of Chemistry,
State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun, Jilin 130012
*Corresponding authors: yulanchen@jlu.edu.cn; xuhuaping@mail.tsinghua.edu.cn
Cite this: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Unlike biological materials that can sense mechanical which provide the functions of stress reporting,
force and actively remodel locally, synthetic polymers mechano-healing, and mechano-remodeling for the
typically break down under stress. Molecular-level deformed film. This study not only illuminates
responses to damage with both stress-reporting and the mechano-responsive nature of Se–Se bonds in the
self-strengthening functions are significant yet diffi- bulk state but also paves the way for the development
cult to realize for synthetic polymers. To overcome of new stress-responsive materials.
this challenge, chemo-mechanical coupling into poly-
mers that can simultaneously ameliorate mechanical,
optical, or other functional properties of a polymer
combined with mechanical treatment will offer a new
principle for materials design. Here, we report a kind
of elastomer in which destructive forces are chan-
nelled into productive and bond-forming reactions by
using diselenide (Se–Se) as a mechanophore. Polyure-
thane has been functionalized with labile Se–Se
bonds, whose mechanical activation generates seleno
radicals that trigger radical transfer and cross-linking
reactions in situ. These reactions are activated effi-
ciently in a mechanical way by compression in bulk Keywords: diselenide, mechano-responsive poly-
materials. The resulting covalent networks possess mers, self-strengthening, polymer mechanochemistry,
turn-on mechano-fluorescence and increased moduli, stress sensing
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
1RESEARCH ARTICLE
Introduction
Early in the 1930s, Staudinger and coworkers observed
the molecular response of synthetic polymeric materials
to applied mechanical force,1–4 which was later on illu-
minated as the mechano-degradation via hemolytic
scission of C–C covalent bonds along the polymer back-
bone. This historical knowledge laid the foundation of
polymer mechano-chemistry, yet at the same time, it
supported the bias that mechanical force was destruc-
tive and associated primarily with polymer degradation
and mechanical failure.5–7 In recent years, such a view
of mechano-chemical reactions has been redirected,
benefiting enormously from the success in design and
synthesis of versatile mechanically activated functional Scheme 1 | Schematic illustration of the mechanical na-
groups (“mechanophores”). Up to now, the productive ture of damage-reporting and self-strengthening func-
use of mechanical force in polymer science has included tions in this work.
mechanically induced color/fluorescence change,8–14
mechano-chemiluminescence,15–19 mechanical release of
small molecules,20–24 mechano-catalysts,25–27 and so on. as useful reagents in free radical reactions.41–43 Recently,
These advances have set the stage for the development we also demonstrated that in the solution state, the dis-
of stress-reporting and self-repairing materials using elenide-centered polymers were mechano-responsive.
mechano-responsive polymers. Both osmotic pressure and shear force under sonication
Elastomers are the functional material of choice for could trigger the breakage of the Se–Se bond to generate
organic electronics, biological scaffolds, sensors, and so polymeric seleno radicals.44,45 Given the relatively low
on. In such scenarios, developing durable elastomers that bond energy of Se–Se bond (172 kJ mol−1) and the high
can sensitively detect mechanical damage and at the reaction efficiency of many seleno radicals,46 in this con-
same strengthen their mechanical properties under me- tribution, we explored the mechano-chemical behaviours
chanical loading is of fundamental importance, yet of the Se–Se bond containing polymers and the resulting
remains a challenge. For this purpose, stress must activate seleno radicals in the bulk state. A new type of mechano-
a bond-forming reaction, for example, creating a new sensitive linear and cross-linked polyurethanes that con-
cross-linking prior to, or immediately following and out- tained Se–Se moieties in the main chain and methyl
pacing chain scission with detectable output.28 Pioneering methacryloyl groups in the side chains were prepared.
work on mechanochemical cross-linking was reported These elastomers were able to damage-report and self-
by Craig et al.,29 based on the ultrasound-induced ring- strengthen synergistically under mechanical loading. The
opening reaction of gem-dibromocyclopropane and mechanical nature of the dual functions was uncovered
subsequent nucleophilic substitution with a bifunctional because it was based on the radical transfer reaction and
carboxylate. Later on, a progression on constructive cross-linking of methyl methacrylate monomers initiated
bond formation, particularly shifting from solution to by the mechanically dissociated Se radicals (Scheme 1).
polymer blends and gels, was rapidly achieved by These findings can promote the practical usage of Se–Se
Sijbesma, Gong and others.27,30,31 Very recently, Otsuka containing elastomers as structural materials.
et al. developed mechano-responsive polyurethanes
with the radical generated from difluorenylsuccinoni-
trile as the mechanophore, which exhibited mechano- Experimental Methods
chromic and self-strengthening functions.32 Up to now,
General
mechanophores that are powerful enough for both
stress-reporting and self-strengthening have been lim- All solvents and reagents were purchased from Sigma-
ited. More labile, dual-functional mechanophores appli- Aldrich (St. Louis, MO, United States), TCI (Tokyo, Japan) ,
cable in the bulk state are highly desirable. or Adamas (Shanghai, China) and used without further
The diselenide bond (Se–Se) is an important dynamic purification, unless otherwise noted. Di-(1-hydroxylunde-
covalent bond that can undergo the metathesis reaction cyl) diselenide (DSe-diol) was synthesized according to
with the generation of seleno radicals under stimuli such previously published methods,1 and 5,6-dihydroxyhexyl
as pH, temperature, light, and so on.33–40 For a long time, methacrylate (DHMA) was also synthesized according to
small organic selenium compounds have been well known previously published methods.2,3 1,4-Butanediol (BDO)
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
2RESEARCH ARTICLE
was purified by dehydration over anhydrous magnesium from methacryloyl radicals, the exact g value could not
sulfate and by distillation under reduced pressure. Poly- be calculated.
tetramethylene glycol (PTMG: Mn = 1000 g/mol) was dried
at 80 °C under vacuum for 2 h before use. Dimethylfor- Fluorescent spectroscopy
mamide (DMF) was dried with CaH2 and purified by vac-
uum distillation, then stored with 4A molecular sieves. All The measurements were carried out using before and
reactions were performed under an argon atmosphere after compression of each polyurethane (PU) film. The
unless otherwise specified, and all glassware was oven- excitation wavelength at 365 nm was selected, and the
dried before use. fluorescence emission peaks were observed at 570 and
605 nm.
Representative polymerize procedure
Taking PU-1 for example: A solution of DSe-diol (127 mg,
Atomic force microscopy study
0.253 mmol), methylenediphenyl diisocyanate (MDI) Atomic force microscopy (AFM) imaging experiments
(930 mg, 3.720 mmol) and dibutyltin dilaurate (5 μL) in were carried out on a commercial AFM (JPK Nanowizard
anhydrous DMF (10 mL) was stirred at 35 °C for 1 h. Dry IV, Berlin, Germany) in quantitative imaging (QI) mode.
PTMG (Mn = 1000 g/mol, 510 mg, 0.510 mmol) and DHMA Before the AFM test, the compressed part and uncom-
(209 mg, 1.035 mmol) in anhydrous DMF (10 mL) was pressed part of the sample were cut off and glued on with
added to this mixture and further stirred at room temper- a clean glass slide via epoxy resin. The silicon AFM probe
ature for 16 h. Then, BDO (175 mg, 1.940 mmol) was added (Olympus, AC-160, Japan, stiffness coefficient 40 N/m,
to the mixture under N2, and the mixture was stirred for probe tip radius 5 nm) was used to randomly select three
24 h at room temperature. The reaction was stopped by regions (the resolution of each region was 128*128). The
adding 0.5 mL of methanol. The crude product was puri- image data and Young’s modulus were analyzed using
fied by precipitation in methanol three times, washing JPK (JPK Instruments AG, German) data processing
with hexane and drying in vacuo. The product was dis- software.
solved in tetrahydrofuran (THF), and the solution was cast
to give PU-1 film.
Results and Discussion
Compression test Design and preparation of diselenide
Films were compressed by using Powder press PC-15 containing elastomer
(Tianguang, Tianjin, China). All processing was done at First, the reactivity of selenium radicals toward acrylate
room temperature in ambient air conditions. monomers was screened. According to a series of control
experiments in solution triggered by photoirradiation, we
Rheometry found, among different diselenide derivatives, that an alkyl
group-modified seleno radical generated from DSe-diol
The rheological properties were measured using an os-
possessed good reactivity47 and was an ideal candidate as
cillatory rheometer (TA Rheometrics, DHR-2; TA Instru-
the initiator for radical polymerization of methyl methac-
ments, United States) equipped with an 8 mm parallel
rylate (experimental details can be found in the supple-
plate-plate geometry. Prior to the experiments, ca. 800–
mental experimental procedures, Supporting Information
2000 μm thick film samples were prepared, and each film
Figure S1). Based on this knowledge, we then developed a
sample was placed between the parallel plates. Storage
kind of segmented PU elastomer (PU-1, Mn = 18.7 kDa) with
moduli were determined at 10 Hz.
bis-undecyl-substituted diselenides (DSe-diol) incorpo-
rated into the main chain and polymerizable side chains
Electron paramagnetic resonance study (Figure 1). The Se–Se moieties could act as the latent
3 mm × 3 mm films of PU-1, PU-2, PU-3, and PU-4 were initiator which then afforded selenol radicals upon me-
prepared. Each film was compressed by a Powder press chanical stimuli and initiated radical polymerization. Be-
PC-15. The compressed samples were transferred into an sides, the methyl methacryloyl units were fixed as
electron paramagnetic resonance (EPR) glass capillary the side groups with the expectation that an efficient
without degassing. EPR measurements were carried out cross-linking reaction could be triggered, leading to a
on a Bruker EMXPLUS Spectrometer (Karlsruhe, Ger- remarkable change in the physical properties of the
many). The spectra of compressed samples were mea- material. Experimentally, DSe-diol, DHMA, PTMG-1000
sured using a microwave power of 2 mW and a field (Mn = 1000 g/mol), and methylenediphenyl diisocyanate
modulation of 0.1 mT with a time constant of 0.03 s and a (MDI) reacted first. The subsequent formation
sweep rate of 0.375 mT/s at 25 °C. Since a Mn marker was of a hard segment was achieved by adding the chain
not used because it overlapped with spectra originated extender BDO to the prepolymer solution to afford PU-1
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
3RESEARCH ARTICLE
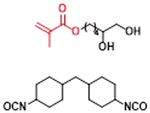
Figure 1 | (a) Monomers used to synthesize the segmented polyurethanes. (b) One possible sequence of blocks in
linear PU-1 and cross-linked PU-6.
(experimental details can be found in the supplemental 365 nm UV irradiation (Figure 2c). Such turn-on fluores-
experimental procedures, Supporting Information Table cence was attractive, since the stressed region could be
S1). Bulk films were then prepared via solution-casting in vividly observed. Not only at the macroscopic scale, the
Teflon molds and subsequent drying for 1 day in vacuo. jointly improved mechanical and optical properties were
sensitive enough to be detected in microregions. As illus-
Mechano-activation of elastomers with self- trated in Figure 2d, we stamped a designed mould on the
reporting and self-strengthening characters film by hand, and under UV irradiation, the corresponding
orange pattern (letters of “TJU”) was observed. Namely,
Compression tests were conducted on the PU-1 film to
mechanically self-reporting to ‘at-risk’ regions was possi-
investigate its mechanically responsive activity. After
ble. We then analyzed the patterned film using AFM
imposition of ca. 400 MPa pressure for 5 min, the com-
quantitative imaging (QI mode), which showed that the
pressed films were soaked in THF for 1 day. As shown in
Young’s modulus of the sample presented a bimodal
Figure 2a, the soluble pristine linear PU turned into an
distribution. According to Figure 2e, the compressed part
insoluble film. Later, more quantitative analyses of the
was stiffer with a larger Young’s modulus (the proportion
mechanical properties before and after compression
of the second peak increased), which was different from
were performed. As shown in Figures 2a and 2b, with
that of the uncompressed area. The two-dimensional dis-
the increase of compression cycles, both the gel fraction
tribution maps of the Young’s modulus (Figure 2f) for the
(see supplemental experimental details) and storage
uncompressed and compressed parts also illustrated the
moduli (Gʹ) increased gradually, indicating that the me-
same results.
chanical properties of the resulting films were depen-
dent on the magnitude of the exerted mechanical force.
Mechanistic study
After 15 cycles, ca. 4.2 folds of the enhancement of Gʹ
with ca. 10% gel fraction were achieved. These results EPR studies of the PU-1 film revealed the presence of
clearly demonstrated that the cross-linking reaction multiple types of radicals after compression. As shown
took place and could effectively strengthen the Se–Se in Figure 3a, in contrast to the pristine film that was EPR
bond-containing elastomers. silent, the selenium radical, the radical derived from
Apart from its mechanical property, the optical property methyl methacrylate48–50 and the diphenylmethyl radi-
of the film was also changed after compression. The as- cal51,52 were distinctly detected in the compressed film. A
prepared PU-1 film was almost nonfluorescent, whereas similar phenomenon has been reported by Flinn, Otsuka,
after compression, it exhibited orange fluorescence under and others.32,52 They all found that the diphenylmethyl
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
4RESEARCH ARTICLE
Figure 2 | (a) Gel fraction and (b) storage modulus as a function of compression cycles of the compressed PU-1 fil
(Data represent average and standard deviation from three parallel experiments for each sample. Insert: Images of PU-
1 film before and after compression and subsequent soaking in THF.) (c) Fluorescence spectra of PU-1 film before and
after compression (Insert: Images of the corresponding films under 365 nm UV light). (d) Images under 365 nm UV
light of the PU-1 film before and after compression using a TJU-shaped metal mold. (e) AFM Young’s modulus
histograms and (f) two-dimensional distribution maps of Young’s modulus for uncompressed part and compressed
part of PU-1.
structure had a great tendency to afford fluorescent control polymer films (PU-2, PU-3, PU-4, PU-5, Supporting
radicals via radical transfer reactions, for example, with Information Figure S2) were prepared. As summarized in
the benzylic hydrogen radicals extracted from the Table 1, they were PU-1 analogs polymerized by changing
diphenylmethyl units. In the present case, owing to the one of the monomers, for example, using dicyclohexyl-
appreciable lability of the Se–Se bond, PU-1 experienced methane-4,4ʹ-diisocyanate instead of MDI (PU-2), or BDO
mechano-chemical transition primarily initiated by the instead of DSe-diol (PU-3), or PTMG instead of DHMA
Se-centered radical mechanism. And the presence of (PU-4). And PU-5 was the physically mixed film. The
methacrylate and diphenylmethyl radicals indicated mechano-responsive behaviours of the four samples were
that the cross-linking reaction proceeded smoothly different from PU-1 (Figure 4a). In particular, the force-
through radical propagation and the transfer steps, induced cross-linking reaction took place in the com-
respectively. Furthermore, it was found that the fluores- pressed PU-2 film (Figure 4b). The storage modulus and
cence intensity decreased gradually and was quenched gel fraction of PU-2 increased alongside the compression
completely after about 3 h. Such observation was most cycles (Figure 4c and Supporting Information Figure S3),
likely attributed to radical quenching over time. Accord- suggesting the resemblance of its self-strengthening abil-
ing to these analyses, we inferred the plausible mecha- ity to PU-1 with increased cross-linking density under
nism as mechanical stress-induced generation of selenol force. As for PU-3, PU-4, and the physically mixed film
radicals to initiate the cross-linking reaction of methac- PU-5, no significant changes in their mechanical proper-
rylate groups, accompanied with turn-on fluorescence ties were observed. Except for PU-4, all the other de-
from the diphenylmethyl radical formed via a radical formed films were nonfluorescent, incapable of damage
transfer pathway (Figure 3b). reporting (Figures 4d–4g and Table 1). Supporting Infor-
To shed more light on the mechanical nature of these mation Figure S4 shows the EPR spectra of these
fluorescent and self-strengthening functions, a series of compressed control films. The signals were different from
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
5RESEARCH ARTICLE
attached MDI moieties were another necessity for
mechano-fluorescence; and (3) the inactivity of the
cross-linking reaction and fluorescence for the physical
counterpart on the other hand showed that the self-
strengthening and self-reporting functions for PU-1 were
indeed triggered by mechanical force. Overall, results
from these control experiments were in good accord with
the mechanism proposed in Figures 3b and 4a, where Se–
Se, MDI, and methyl methacrylate units incorporated in
polymer chains served as the latent initiator, stress-report-
er, and monomer for self-reporting and self-strengthening
abilities, respectively.
Mechano-healing and mechano-remodeling
of fractured elastomers
More practically, this selenol radical-involved cross-linking
system was then demonstrated as sensitive and powerful
enough for mechano-healing and mechano-remodeling of
damaged PU samples. As illustrated in Figure 5a, the
broken linear polymer PU-1 was healed into a uniform film
after mild compression. The resulting film was insoluble
due to the stress-induced cross-linking reactions. Notably,
even for cross-linked PU networks that were in principle
difficult to be processed, remodeling worked well under
mechanical treatment. To demonstrate, cross-linked PU-6
containing DSe-diol and DHMA in the polymer chains and
triethanolamine as the cross-linker was synthesized (Fig-
ure 1). When PU-6 was first cut into small pieces followed
by compression, an intact cross-linked film could be
afforded (Figure 5b). The shape of the reformed film could
Figure 3 | (a) EPR spectrum of the compressed film of be remolded with the aid of different shapes and sizes of
PU-1 (g = 2.00295). (b) Schematic illustration of the mech- metal molds (Figure 5c). Meanwhile, the compressed re-
anism of force-induced fluorescent radical transfer and gion exhibited orange fluorescence. In this sense, the
cross-linking reactions with Se–Se as the mechanophore. destructive effect of mechanical force on bulk films can
effectively be shifted to productive dual functions.
that of PU-1, exhibiting either only two of the three types
of radicals or being free of radicals.
Conclusion
Valuable information was obtained from these control
experiments: (1) Se–Se, methyl methacrylate units, and To conclude, we have developed a new kind of Se–Se
their covalent linking into polymer chains were essential containing elastomers with both stress-reporting and
factors for cross-linking reactions; (2) the covalently self-strengthening characteristics. For the first time, the
Table 1 | Key Components of the PU Samples and Their Ability to Undergo Force-Induced Cross-Linking and
Fluorescence
Samples PU-1 PU-2 PU-3 PU-4 PU-5
Cross-linking Yes Yes No No No
Fluorescence Yes No No Yes No
Components PU-3
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
6RESEARCH ARTICLE
Figure 4 | (a) Schematic illustration of possibly occurring force-induced fluorescent radical transfer and/or cross-
linking reactions for control polymers PU-2, PU-3, and PU-4. (b) Images of PU-2, PU-3, PU-4, and PU-5 films before and
after compression and subsequent soak in THF. (c) The storage modulus of controlled PUs before and after
compression. Fluorescence spectra of (d) PU-2, (e) PU-3, (f) PU-4, and (g) PU-5 before and after compression
(Insert: Images of the corresponding films under 365 nm UV light).
mechano-chemical activation of the Se–Se unit was be achieved in a green and facile way, simply by com-
realized in the bulk state. Benefiting from the low bond pression of the bulk materials. Moreover, the main chain
energy of the Se–Se bond, polymeric seleno radical incorporating diphenylmethyl moieties experienced a
could be produced mechanically and were reactive radical transfer reaction, affording fluorescent radicals
enough to initiate the cross-linking reaction of methyl to self-report the excessive stress imposed on the de-
methacrylate side chains of the PU. In this way, formed film. Notably, these molecular-level responses
mechano-healing and mechano-remodeling of both and their impact on polymer mechanical and optical
linear and cross-linked PUs with increased moduli can properties resembled biological materials to the extent
that were intelligent enough to sense the damage and
actively remodel locally. Such dual functions, not only in
biological systems but also in artificial materials, are criti-
cal for their practical usage. The research presented here
thus is an important step toward functional Se-containing
durable elastomers. Also, our work enriches the polymer
design strategy for productive usage of mechanical force.
Supporting Information
Supporting Information is available and includes experi-
ments, characterization, and supplementary figures and
table.
Figure 5 | Images showing (a) mechano-healing process Conflict of Interest
of PU-1; (b and c) mechano-remodeling process of PU-6
into different shapes. There is no conflict of interest to report.
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
7RESEARCH ARTICLE
13. Wu, M.; Guo, Z.; He, W.; Yuan, W.; Chen, Y. Empowering
Funding Information Self-Reporting Polymer Blends with Orthogonal Optical
Properties Responsive in a Broader Force Range. Chem. Sci.
The financial support of this research by the National
2021, 12, 1245–1250.
Natural Science Foundation of China (grant nos.
14. Imato, K.; Irie, A.; Kosuge, T.; Ohishi, T.; Nishihara, M.;
21734006 and 21975178) and the National Key Research
Takahara, A.; Otsuka, H. Mechanophores with a Reversible
and Development Program of China (grant nos.
Radical System and Freezing-Induced Mechanochemistry in
2017YFA0207800 and 2017YFA0204503) is gratefully Polymer Solutions and Gels. Angew. Chem. Int. Ed. 2015, 54,
acknowledged. 6168–6172.
15. Chen, Y.; Spiering, A. J.; Karthikeyan, S.; Peters, G. W.;
Meijer, E. W.; Sijbesma, R. P. Mechanically Induced Chemilu-
References minescence from Polymers Incorporating a 1,2-Dioxetane
Unit in the Main Chain. Nat. Chem. 2012, 4, 559–562.
1. Staudinger, H.; Bondy, H. F. Über isopren und kautschuk,
16. Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R. P.; Creton,
19. Mitteil.: Über die molekülgröße des kautschuks und der
C. Toughening Elastomers with Sacrificial Bonds and Watch-
balata. Ber. Dtsch. Chem. Ges. 1930, 63, 734–736.
ing Them Break. Science 2014, 344, 186–189.
2. Staudinger, H.; Heuer, W. Über hochpolymere
17. Clough, J. M.; Balan, A.; van Daal, T. L. J.; Sijbesma, R. P.
verbindungen, 93. Mitteil.: Über das zerreißen der faden-
Probing Force with Mechanobase-Induced Chemilumines-
moleküle des poly-styrols. Ber. Dtsch. Chem. Ges. 1934, 67,
cence. Angew. Chem. Int. Ed. 2016, 55, 1445–1449.
1159–1164.
3. Staudinger, H.; Leupold, E. O. Über isopren und 18. Deng, Y.; Yuan, Y.; Chen, Y. Covalently Cross-Linked
kautschuk, 18. Mitteil.: Viscositäts-unter-suchungen an bala- and Mechanochemiluminescent Polyolefins Capable of Self-
ta. Ber. Dtsch. Chem. Ges. 1930, 63, 730–733. Healing and Self-Reporting. CCS Chem. 2020, 2, 1316–1324.
4. Sohma, J. Mechanochemistry of Polymers. Prog. Polym. 19. Li, X.; Li, J.; Wei, W.; Yang, F.; Wu, M.; Wu, Q.; Xie, T.;
Sci. 1989, 14, 451–596. Chen, Y. Enhanced Mechanochemiluminescence from End-
5. Lin, Y.; Kouznetsova, T. B.; Craig, S. L. Mechanically Functionalized Polyurethanes with Multiple Hydrogen
Gated Degradable Polymers. J. Am. Chem. Soc. 2020, 142, Bonds. Macromolecules 2021, 54, 1557–156.
2105–2109. 20. Versaw, B. A.; Zeng, T.; Hu, X.; Robb, M. J. Harnessing the
6. Lu, Y.; Sugita, H.; Mikami, K.; Aoki, D.; Otsuka, H. Mecha- Power of Force: Development of Mechanophores for Molec-
nochemical Reactions of Bis(9-methylphenyl-9-fluorenyl) ular Release. J. Am. Chem. Soc. 2021, 143, 21461–21473.
Peroxides and Their Applications in Cross-Linked Polymers. 21. Diesendruck, C. E.; Steinberg, B. D.; Sugai, N.; Silber-
J. Am. Chem. Soc. 2021, 143, 17744–17750. stein, M. N.; Sottos, N. R.; White, S. R.; Braun, P. V.; Moore, J. S.
7. Giannantonio, M. D.; Ayer, M. A.; Verde-Sesto, E.; Lat- Proton-Coupled Mechanochemical Transduction: A
tuada, M.; Weder, C.; Fromm, K. M. Triggered Metal Ion Mechanogenerated Acid. J. Am. Chem. Soc. 2012, 134,
Release and Oxidation: Ferrocene as a Mechanophore in 12446–12449.
Polymers. Angew. Chem. Int. Ed. 2018, 57, 11445–11450. 22. Larsen, M. B.; Boydston, A. J. “Flex-Activated” Mechan-
8. Davis, D. A.; Hamilton, A.; Yang, J.; Cremar, L. D.; Gough, ophores: Using Polymer Mechanochemistry to Direct
D. V.; Potisek, S. L.; Ong, M. T.; Braun, P. V.; Martínez, T. J.; Bond Bending Activation. J. Am. Chem. Soc. 2013, 135,
White, S. R.; Moore, J. S.; Sottos, N. R. Force-Induced Acti- 8189–8192.
vation of Covalent Bonds in Mechanoresponsive Polymeric 23. Sha, Y.; Zhang, Y.; Xu, E.; Wang, Z.; Zhu, T.; Craig, S. L.;
Materials. Nature 2009, 459, 68–72. Tang, C. Quantitative and Mechanistic Mechanochemistry in
9. Hemmer, J. R.; Rader, C.; Wilts, B. D.; Weder, C.; Ferrocene Dissociation. ACS Macro Lett. 2018, 7, 1174–1179.
Berrocal, J. A. Heterolytic Bond Cleavage in a Scissile 24. Hu, X.; Zeng, T.; Husic, C. C.; Robb, M. J. Mechanically
Triarylmethane Mechanophore. J. Am. Chem. Soc. 2021, Triggered Small Molecule Release from a Masked Furfuryl
143, 18859–18863. Carbonate. J. Am. Chem. Soc. 2019, 141, 15018–15023.
10. McFadden, M. E.; Robb, M. J. Force-Dependent Multi- 25. Piermattei, A.; Karthikeyan, S.; Sijbesma, R. P. Activating
color Mechanochromism from a Single Mechanophore. J. Catalysts with Mechanical Force. Nat. Chem. 2009, 1, 133–137.
Am. Chem. Soc. 2019, 141, 11388–11392. 26. Michael, P.; Binder, W. H. A Mechanochemically
11. Qi, Q.; Sekhon, G.; Chandradat, R.; Ofodum, N. M.; Shen, Triggered “Click” Catalyst. Angew. Chem. Int. Ed. 2015, 54,
T.; Scrimgeour, J.; Joy, M.; Wriedt, M.; Jayathirtha, M.; Darie, C. 13918–13922.
C.; Shipp, D. A.; Liu, X.; Lu, X. Force-Induced Near-Infrared 27. Jakobs, R. T. M.; Ma, S.; Sijbesma, R. P. Mechanocatalytic
Chromism of Mechanophore-Linked Polymers. J. Am. Chem. Polymerization and Cross-Linking in a Polymeric Matrix. ACS
Soc. 2021, 143, 17337–17343. Macro Lett. 2013, 2, 613–616.
12. Muramatsu, T.; Okado, Y.; Traeger, H.; Schrettl, S.; 28. Black, A. L.; Lenhardt, J. M.; Craig, S. L. From Molecular
Tamaoki, N.; Weder, C.; Sagara, Y. Rotaxane-Based Mechanochemistry to Stress-Responsive Materials. J. Mater.
Dual Function Mechanophores Exhibiting Reversible and Chem. 2011, 21, 1655–1663.
Irreversible Responses. J. Am. Chem. Soc. 2021, 143, 9884– 29. Ramirez, A. L. B.; Kean, Z. S.; Orlicki, J. A.; Champhekar,
9892. M.; Elsakr, S. M.; Krause, W. E.; Craig, S. L. Mechanochemical
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
8RESEARCH ARTICLE
Strengthening of a Synthetic Polymer in Response to Typi- Radical Polymerization of Styrene Under UV Irradiation. J.
cally Destructive Shear Forces. Nat. Chem. 2013, 5, 757–761. Polym. Sci., Part A: Polym. Chem. 2012, 50, 2211–2218.
30. Zhang, H.; Gao, F.; Cao, X.; Li, Y.; Xu, Y.; Weng, W.; 42. Wu, J.; Ding, C.; Xing, D.; Zhang, Z.; Huang, X.; Zhu, X.; Pan,
Boulatov, R. Mechanochromism and Mechanical-Force- X.; Zhu, J. The Functionalization of Poly(ɛ-caprolactone) as a
Triggered Cross-Linking from a Single Reactive Moiety In- Versatile Platform Using ɛ-(α-Phenylseleno) Caprolactone as a
corporated into Polymer Chains. Angew. Chem. Int. Ed. 2016, Monomer. Polym. Chem. 2019, 10, 3851–3858.
55, 3040–3044. 43. Lin, X.; Li, J.; Zhang, J.; Liu, S.; Lin, X.; Pan, X.; Zhu, J.; Zhu,
31. Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; X. Living Cationic Polymerization of Vinyl Ethers Initiated by
Gong, J. P. Mechanoresponsive Self-Growing Hydrogels Electrophilic Selenium Reagents Under Ambient Condition.
Inspired by Muscle Training. Science 2019, 363, 504–508. Polym. Chem. 2021, 12, 983–990.
32. Seshimo, K.; Sakai, H.; Watabe, T.; Aoki, D.; Sugita, H.; 44. Xia, J.; Zhao, P.; Pan, S.; Xu, H. Diselenide-Containing
Mikami, K.; Mao, Y.; Ishigami, A.; Nishitsuji, S.; Kurose, T.; Ito, Polymeric Vesicles with Osmotic Pressure Response. ACS
H.; Otsuka, H. Segmented Polyurethane Elastomers with Macro Lett. 2019, 8, 629–633.
Mechanochromic and Self-Strengthening Functions. Angew. 45. Wu, Q.; Yuan, Y.; Chen, F.; Sun, C.; Xu, H.; Chen, Y.
Chem. Int. Ed. 2021, 60, 8406–8409. Diselenide-Linked Polymers Under Sonication. ACS Macro
33. Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Lett. 2020, 9, 1547–1551.
Responsive Assemblies Formed from Diselenide Block 46. Kildahl, N. K. Bond Energy Data Summarized. J. Chem.
Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. Educ. 1995, 72, 423–424.
34. Cao, W.; Zhang, X.; Miao, X.; Yang, Z.; Xu, H. 47. Kang, X.; Yuan, Y.; Xu, H.; Chen, Y. Thermal- and Light-
γ-Ray-Responsive Supramolecular Hydrogel Based on a Driven Metathesis Reactions Between Different Diselenides.
Diselenide-Containing Polymer and a Peptide. Angew. Chem. Res. Chin. Univ. 2021. DOI: https://doi.org/10.1007/
Chem. Int. Ed. 2013, 52, 6233–6237. s40242-021-1149-8
35. Ji, S.; Cao, W.; Yu, Y.; Xu, H. Dynamic Diselenide Bonds: 48. Truffier-Boutry, D.; Gallez, X. A.; Demoustier-Cham-
Exchange Reaction Induced by Visible Light Without Catal- pagne, S.; Devaux, J.; Mestdagh, M.; Champagne, B.; Leloup,
ysis. Angew. Chem. Int. Ed. 2014, 53, 6781–6785. G. Identification of Free Radicals Trapped in Solid Metha-
36. Ji, S.; Cao, W.; Yu, Y.; Xu, H. Visible-Light-Induced Self- crylated Resins. J. Polym. Sci. Part A: Polym. Chem. 2003, 41,
Healing Diselenide-Containing Polyurethane Elastomer. Adv. 1691–1699.
Mater. 2015, 27, 7740–7745. 49. da Silva Fontes, A.; Vicentin, B. L. S.; Valezi, D. F.;
37. Ji, S.; Xia, J.; Xu, H. Dynamic Chemistry of Selenium: da Costa, M. F.; Sano, W.; Di Mauro, E. A Multifrequency (X-,
Se-N and Se-Se Dynamic Covalent Bonds in Polymeric Sys- Q-, and W-Band) EPR and DFT Study of a Photopolymeriz-
tems. ACS Macro Lett. 2016, 5, 78–82. able Dental Resin. Appl. Magn. Reson. 2014, 45, 681–692.
38. Xia, J.; Li, T.; Lu, C.; Xu, H. Selenium-Containing Poly- 50. Vicentin, B. L. S.; Netto, A. M.; Blümich, B.; Mauro, E. D.
mers: Perspectives toward Diverse Applications in Both Identification of Free Radicals Generated by Different Curing
Adaptive and Biomedical Materials. Macromolecules 2018, Modes in a Dental Resin Cement. Appl. Magn. Reson. 2016,
51, 7435–7455. 47, 1003–1014.
39. Xia, J.; Tan, Y.; Xu, H. Selenium-Containing Dynamic 51. Hirano, T.; Li, W.; Abrams, L.; Krusic, P. J.; Ottaviani, M. F.;
Covalent Polymers. Acta Polym. Sin. 2020, 51, 1190–1200. Turro, N. J. Reversible Oxygenation of a Diphenylmethyl
40. Pan, S.; Yang, J.; Ji, S.; Li, T.; Gao, S.; Sun, C.; Xu, H. Cancer Radical Rendered Supramolecularly Persistent. J. Am. Chem.
Therapy by Targeting Thioredoxin Reductase Based on Se- Soc. 1999, 121, 7170–7171.
lenium-Containing Dynamic Covalent Bond. CCS Chem. 52. Toivola, R.; Jang, S.; Mannikko, D.; Stoll, S.; Jen, A. K.;
2020, 2, 225–235. Flinn, B. D. Mechanochemical Changes in Absorption and
41. Zeng, J.; Zhu, J.; Zhang, Z.; Pan, X.; Zhang, W.; Cheng, Z.; Fluorescence of DDM-Containing Epoxies. Polymer 2018,
Zhu, X. New Selenium-Based Iniferter Agent for Living Free 142, 132–143.
Citation: CCS Chem. 2022, Just Published. DOI: 10.31635/ccschem.022.202201874
Link to VoR: https://doi.org/10.31635/ccschem.022.202201874
9You can also read