ESOCAP CHANGING THE PARADIGM OF EOE TREATMENT - EUROPE SPECIAL
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
www.pharmatechoutlook.com April - 2020 ISSN 2644-2787
EUROPE SPECIAL
DRUG DISCOVERY &
DEVELOPMENT
EDITION
Changing the
Paradigm of
EoE Treatment
EsoCap Isabelle Racamier,
CEO and Board Director
$15COVER STORY
EsoCap
Changing the
Paradigm of EoE
Treatment
M
odern medicine began to emerge in diagnosis and treatment of many of the previously
the post-industrial revolution era of unknown diseases, including eosinophilic
the 18th century. Since those early esophagitis (EoE).
days, medicine has advanced by Earlier, if someone had EoE, the chances of
leaps and bounds, with many scientific discoveries a doctor misdiagnosing it as gastro-esophageal
being made and breakthroughs achieved. Today, reflux disease (GERD) were particularly high. It
technology’s fingerprints can be found all over the was not so long ago that doctors realised that even
healthcare domain—from new ways of teaching though EoE showed symptoms similar to GERD,
medical students to taking care of patients in it was a different, rare disease. In EoE, eosinophils
hospitals with the most sophisticated equipment. (a type of white blood cell) build up in the lining
So much so that technology now helps in accurate of the esophagus. This buildup, often caused by aTOP 10
DRUG DISCOVERY
AND DEVELOPMENT
SOLUTION PROVIDERS IN EUROPE - 2020
We have already reached
a point of achieving
technical development
and technical proof of Isabelle Racamier,
CEO and Board Director
concept of our underlying
technologyperson’s reaction to allergens, inflames gastrointestinal diseases. And to bring to a mouthpiece through a slit in the
the esophageal tissue and leads to this smart drug application technology capsule and a connecting thread. The
difficulty in swallowing and extremely into clinical testing and to EoE patients, mouthpiece is further placed onto
painful food impaction. While the Prof. Dr. Weitschies collaborated with a specially-designed drinking cup,
current treatment options are limited Dr. Werner Tschollar. The result of their which allows the patients to swallow
to strict dietary measures and out of partnership led to the foundation of the capsule easily. When patients
label administration of steroids, there is EsoCap, an emerging biotech startup consume the capsule, the film sticks to
no single globally accepted therapy for based in Basel, Switzerland. Today, the the esophageal mucosa, ensuring local
the condition. Moreover, even though company is building upon the work delivery and prolonged mucosal contact
steroids for EoE are generally effective, of the Greifswald Center of Drug time as the film dissolves slowly over
these medications are minimally
absorbed by the esophageal segment
of the gastrointestinal tract. The short
transit time of the drug from mouth to
the stomach hinders the expected effect
of the drug. Thus, the pharmaceutical
industry had been on the lookout for a
tool that can administer the medications
directly to the esophagus for treating
EoE.
At this juncture, the group of Prof.
Dr. Werner Weitschies, from the Center
of Drug Absorption and Transport at
the University of Greifswald, came up
with a breakthrough that the healthcare
industry has been waiting for so long.
They developed a novel drug delivery
platform for the treatment of upper
Absorption and Transport to develop a time. Further, with an approximate
unique topical drug delivery platform length of 25 cm, the thin film stretches
designed to assist in the treatment of the throughout the length of the esophagus.
upper gastrointestinal tract. Currently Isabelle Racamier, the CEO and board
entering the phase two clinical stage, the member of EsoCap, believes that their
smart drug application technology will topical drug delivery platform comes
allow targeted delivery of a broad range at a time when drug discovery and
of pharmaceutical drugs to the affected development are witnessing the next
areas with maximum accuracy. stage of evolution. She says, “We have
already reached a point of achieving
The Paradigm Shift in EoE technical development and technical
Treatment proof of concept of our underlying
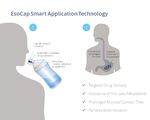
EsoCap’s smart drug delivery platform technology.” Markedly, the University
uses a thin film loaded with the drug of Greifswald, EsoCap’s collaborator
substance. The film is rolled up into in the development of the product, has
a capsule, which, when swallowed, already corroborated the feasibility of
unrolls the film in the esophagus and the drug delivery concept. An imaging
delivers the drug slowly over time. To study showed sustained placement of a
ensure the film stays in the intended polymer film on the esophageal mucosa.
area of application, it is designed such Such a highly localised approach
that one end of the film is attached reduces the risk of side effects sincedoctors do not have to administer any
systemic drug. Therefore, the smart drug
application technology opens the door
for new treatment options that were not
possible up until now because of the
limitations around local delivery and
subsequent side effects of drugs.
Applications beyond EoE
EsoCap’s innovative technology is not
only meant to benefit thousands of
patients with EoE but also be a major
advancement in the treatment of several
diseases of the upper gastrointestinal
tract. In a recent multidisciplinary
scientific advisory meeting held by
EsoCap, the role of its novel technology
for the topical treatment of eosinophilic
esophagitis was discussed. In the
conference, the experts also explored
the broader applications of EsoCap’s
drug delivery platform in the treatment
of other esophageal diseases. Executive technology can easily be combined partners for reaching out to a large scope
teams of many large pharmaceutical with other drugs to treat each of the of functions. These external contributors
companies and other accomplished gastroenterology and oncology disorders’ include companies covering industrial
experts in the field have already started indications. Besides, considering the development, production of different
noticing this innovative drug delivery flexibility of the smart drug application pieces of the drug delivery platform,
platform. “EsoCap’s new platform has technology, it can be used with both along with scientific and regulatory
given pharmaceutical community a clinically proven and innovative advisors. Moreover, both internal and
reason to be excited,” comments Isabelle. new classes of drugs that are still in external members are encouraged to
Incentivised by such positive development. bring in their personal initiative and
reception, EsoCap initiated a
contribute to strategy development,
cooperation with Lohmann Therapie- The Art of Running a Startup and at the same time have an action-
Systeme (LTS), a leader in the The successful journey of EsoCap, so far,
oriented mindset for the execution
production of oral thin films, to begin would not have been possible without
of the company’s business strategy.
the industrial development of the drug the harmonious blend of the expertise
delivery technology. The collaboration According to Isabelle, the beauty of
of each management and board member.
is paving the way for clinical trials being a startup is in the fact that their
“When the team plays together; we
for patients suffering from EoE. The internal functioning resembles that of an
need to be in full harmony with a
potential of the drug delivery platform high quality of execution to reach the orchestra. Isabelle adds, “Everyone needs
has also been recognised by the U.S. business goals of EsoCap,” underlines to come in and out at the right time
Food & Drug Administration (FDA), Isabelle. The management team of with the right component based on their
which has granted EsoCap the Orphan EsoCap has educational backgrounds expertise and talent.”
Drug Designation status in the and past experiences in the fields of Such expertise and interoperability
treatment of EoE. science, management, and finance. have helped EsoCap in finalising the
However, Isabelle notes, “We are And each individual in the team brings development of its product and the
currently starting a phase two clinical their subject matter expertise in general company is currently starting a phase
trial for only one indication of EoE, management, strategic planning, finance, two clinical trial. “The next step for
and there are five additional indications project management, and development EsoCap in the upcoming months will be
of gastroenterology and oncology in the pharmaceutical space. In addition to enter into a transaction with a major
disorders covering approximately 370 to its internal team, EsoCap also biopharmaceutical company,” concludes
million patients.” EsoCap’s drug delivery collaborates with a number of external Isabelle.APRIL - 2020
ISSN 2644-2787
EUROPE EDITION
WWW.PHARMATECHOUTLOOK.COM
EsoCap
TOP
DRUG DISCOVERY
AND DEVELOPMENT
SOLUTION PROVIDERS
IN EUROPE
2020
Recognised by
TECH OUTLOOK
EsoCap
TOP
DRUG DISCOVERY
AND DEVELOPMENT
SOLUTION PROVIDERS
IN EUROPE
2020
Recognised by
TECH OUTLOOK
The annual listing of 10 companies that are at the forefront of providing
Drug Discovery and Development solutions and transforming businessesYou can also read