The cyclooxygenase theory of aspirin-induced asthma
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Eur Resplr J REVIEW
1990, 3, 588-593
The cyclooxygenase theory of aspirin-induced asthma
A. Szczeklik
The cyclooxygenase theory of aspirin-induced asthma. A. Szczeklik Dept of Allergy and Clinical Immunology,
ABSTRACT: Aspirin-Induced asthma Is a distinct clinical syndrome wblcb Copemii:us Academy of Medicine, Skawinska,
affects about 10% of adult asthmatics. In these patients asplrlri and several Cracow, Poland.
other analgesics precipitate asthmatic attacks. The Idea that the attacks
Correspondence: A. Szcuklik, Dept of Allergy and
mlgbt result from the specific lnblbltlon of a single enzyme, namely Clinical Immunology, Copemicus Academy of
cyclooxygenase, has gained both experimental and Clinical support. Medicine, Skawinska 8, 31-066 Cracow, Poland.
It stimulated a number of hypotheses on the mechanism of
bronchoconstrlctlon. All these hypotheses, here discussed, operate within Keywords: Aspirin; aspirin -induced asthma;
the framework of the cyclooxygenase theory. Their major assuniption is cyclooxygenase; eicosanoids.
that inhibition of cyclooxygenase triggers specific biochemical reactions
which lead to open asthma attacks. Received: June 13, 1989; accepted September 7, 1989.
Eur Respir J., 1990, 3, 588-593.
The majority of people tolerate aspirin well. In the early seventies, allergic mechanisms as an
Asthmatics, however, are an exception. In about 10% of explanation for aspirin intolerance were vigorously pur-
adults with asthma, but rarely in asthmatic children, sued. Contrary to these concepts, the cyclooxygenase
aspirin and other non-steroidal anti-inflammatory drugs theory proposed that precipitation of asthmatic attacks
(NSAID) precipitate asthma attacks. This distinct by aspirin is not based on antigen-antibody reactions, but
clinical syndrome is called aspirin-induced asthma (AlA) stems from the pharmacological action of the drug. The
[1-3]. It appeals to pharmacologists, biochemists and original observations [5, 7] that the drug intolerance could
clinicians as a remarkable model for the study of be predicted on the basis of its in vitro inhibition of
mechanisms operating in asthma. cyclooxygenase, have been consistently reaffirmed
Many concepts have been advanced to explain the during the ensuing years [8] . Evidence in favour of the
pathogenesis of AlA [3, 4]. The idea that the attacks cyclooxygenase theory [3] can be summarized as
might result from the specific inhibition of a single follows:
enzyme, namely cyclooxygenase, in the respiratory tract 1) analgesics with anticyclooxygenase activity
has been, perhaps, most discussed. It stimulated a invariably precipitate bronchoconstriction in aspirin-
number of hypotheses o n mechanisms of sensitive patients;
bronchoconstriction. All of these hypotheses operate 2) analgesics not affecting cyclooxygenase are devoid
within the framework of the cyclooxygenase theory. Thus, of bronchospastic properties in these patients;
their major assumption, now rather firmly established, is 3) there is a positive correlation between the potency of
that inhibition of cyclooxygenase triggers specific analgesics to inhibit cyclooxygenase in vitro and their
biochemical reactions which lead to open asthma attacks. potency to induce asthmatic attacks in the sensitive
patients;
4) the degree of enzymatic inhibition that is sufficient to
Formulation of the theory precipitate bronchoconstriction is an individual hallmark
(thus, if the threshold dose for any anticyclooxygenase in
A hypothesis was put forward [5] that in sensitive a particular patient is known, one can predict the
patients, precipitation of asthma attacks by certain anal- threshold doses for other analgesics in that patient);
gesics results from inhibition of cyclooxygenase, leading 5) in vitro anti-cyclooxygenase inhibitors activate plate-
to an imbalance of prostanoids in the respiratory tract. lets to release cytotoxic mediators in aspirin-sensitive
Cyclooxygenase, an atypical lipoxygenase, is present in asthmatics, but not in the atopic asthmatics or healthy
most human tissues, including lungs. It introduces two subjects [9, 10];
molecules of oxygen into arachidonic acid, converting it 6) in patients with AlA inhibition of thromboxane Az
into a prostaglandin peroxide. This is the beginning of (TXA2), next to cyclooxygenase enzyme in the ara-
the metabolic pathway leading to formation of chidonic acid cascade, neither precipitates asthmatic
prostaglandins, thromboxane and prostacyclin. Cyclooxy- attacks nor alters pulmonary function [11];
genase is inhibited by aspirin, and several other 7) after aspirin desensitization, cross-desensitization to
analgesics; a phenomenon which might explain their other analgesics which inhibit cyclooxygenase also
pharmacological action [6). occurs [8].ASPIRIN-INDUCED ASTHMA 589
Thus, the inhibition of bronchial cyclooxygenase by if cyclooxygenase had been inhibited. The definition of
aspirin-like drugs appears to set off a chain of reactions leukotrienes suggested that they would provide potent
leading to asthma attacks in aspirin-intolerant patients. mediation of neutrophil intlux into the tissue via the action
What follows at the biochemical level remains largely of leukotriene B4 (LTB4), and a potent stimulation for
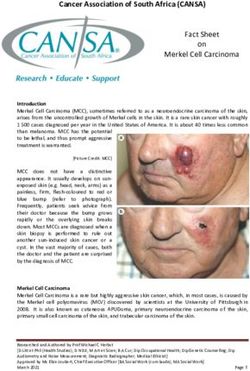
unknown, (fig. 1). bronchoconstriction, mucosal penneability and mucus
secretion by the actions of leukotriene C4 (LTC4), leukot-
12-HETE, riene D4 (LTD 4 ), and leukotriene E 4 (LTE 4 ). The
4_,.o,,.~ b ) explanation for aspirin-induced asthma was then
postulated as simply being caused by shunting of
PGH2 4 / r-- AA I'@ 11 LTA4 __. LTB4 arachidonic acid from the generation of prostaglandins to
+
~',,,~',, LTC4 the biosynthesis of leukotrienes. In a simplified way, it
would be a redirection of flow in a bifurcated vessel:
LTD4
from the blocked arm to an open one. More sophisticated
LTE4 explanations have been put forward. Biosynthesis of
' '._. Cytotoxic leukotrienes could be enhanced by overproduction of
mediator 12-hydroperoxyeicosatetraenoic acid (12-HPETE) [14] or
·trom platelets removal of inhibiting control of PGEJPG!z [15]. Both
possibilites are a logical consequence of cyclooxygenase
pathway inhibition.
There is some experimental support for the concept of
shift in arachidonic acid metabolism, though clinical
evidence is still lacking. In a guinea-pig model of
Fig. 1. - Aspirin-induced astluna hypothetical alterations in arachidonic antigen-induced anaphylaxis, pretreatment of animals with
acid metabolism following inhibition of cyclooxygenase by aspirin.
AA: arachidonic acid; COX: cyclooxygenase; LOX: lipoxygcnase; indomethacin resulted in an augmentation of the
PGH2: cyclic cndoperoxide !1~: PGI~: p~tacyclin; PGs: prosta~~;tandins; pulmonary mechanical response to intravenous antigen
TXA1 : thromboxane A.,; LTA 4 : leukolncnc Ai._LTB 4 : lcukoLnene B,; and this was accompanied by an increased generation of
LTC,: leukotricoe C,; L'l'D4 : leukotrienc 0 1; LTH4: leukotricne E4; 12- LTB4 [16]. In antigen-challenged sheep lung in vivo,
[IIZTE: 12-hydroperoxyeicosatetraenoic ac1d; IS-HETE: JS-hydroper-
oxyeicosatetraenoic acid; LTX: lipoxin A; LXB: lipoxin B. PGE 2 : cyclooxygenase inhibition enhanced leukotriene produc-
prostaglandin E2; PGF,..: prostaglandin F,... tion [17). Pretreatment of passively sensitized human
airways with indomethacin resulted ~n an increased
release of leukotrienes from human bronchi in response
Early explanations to both antigen and anti-IgE stimulation [18]. However,
others have found (19] that in normal human parenchyma
At the time of publication of the cyclooxygenase hy- an anti-IgE challenge in the presence of indomethacin
pothesis [5), the only eicosanoids known to be produced does not produce a shift towards leukotriene formation.
by the cells of the respiratory tract were prostaglandin E2 Two groups studied the release of leukotrienes into the
(PGE2) and prostglandin F,.. (PGF2«). It was presumed nasal cavity following aspirin administration to patients
(12), therefore, that although cyclooxygenase was inhib- with AlA. 0RTOLANI et al. [20] noticed an increase in
ited by aspirin, a selective deficiency among the mean LTC4 concentration in nasal washings of 7 aspirin-
cyclooxygenase products must also occur, such that sensitive asthmatics following nasal spray provocation
bronchodilator PGE 2 was decreased relative to bron- with aspirin. However, clinical symptoms occurred within
choconstrictor PGF2o:. This idea was not universally 1-2 min of the challenge, while LTC4 increase was
appealing because, although the efficacy of aspirin and observed 60 min later. FERRERI et al. [21] used oral
certain other NSAIDs as inhibitors of cyclooxygenase aspirin to provoke clinical symptoms in 5 intolerant
was clearly demonstrated, relative effects on (PGH2)-PGE2 patients. During the provoked reactions, LTC4 increased
isomerase and PGHz-PGFZ« isomerase were not. in 3 patients. In 2 of the 5 patients a fall in PGE2 pre-
An early explanation (5], that aspirin-sensitive ceded appearance of clinical symptoms: In the control
asthmatics might rely more on PGE 2 than on the subjects, ingestion of higher doses of aspirin (650 mg)
~-adrenergic system to keep their bronchi unobstructed, resulted in a distinct fall in PGE2 without the release of
seems unlikely today. These patients, indeed, respond LTC4 into nasal washings. A recent study by BISGAARD et
better to inhaled PGE 2 than do other asthmatics [I 3] but al. [22) has cast serious doubt on the validity of media-
clinical practice leaves no doubt that their lung function tor measurement in nasal lavage in relation to symptoms
also improves substantially following inhalation of following local nasal challenge.
~-mime tics. It is not clear in which cells of the respiratory tract
alterations in arachidonic acid metabolism might occur.
Leucocytes, especially eosinophils, present in large
Participation of leukotrienes amounts in nasal and bronchial tissue of aspirin-sensitive
asthmatics [3, 23] could be considered as a source of
In 1979, when leukotrienes were discovered, it became leukotrienes. GoETZL et al. [24] suggested a generalized
apparent that arachidonic acid could be diverted from the abnormality of the regulation of arachidonic acid oxida-
cyclooxygenase pathway to the 5-lipoxygenase pathway, tive pathways in peripheral blood leucocytes of patients590 A. SZCZEKLIK
with AlA. Two recent studies do not support this the inhibitor, administered by inhalation remained,
idea. NrZANKOWSKA et al. [25) studied production however, uncertain.
by polymorphonuclear leucocytes of 5-dehydroper-
oxyeicosatetraenoic acid (5-HETE) and LTB4 in 10
aspirin-sensitive asthmatics and 10 matched healthy Platelet involvement
controls. The blood cells were obtained before admini-
stration of the threshold doses of aspirin, and during the In the last few years attention has been paid to
aspirin-induced reactions. Initial levels of eicosanoids possible participation of platelets in pathogenesis of
determined did not differ between the two groups, and bronchial asthma [34, 35], particularly in aspirin-induced
remained unchanged following aspirin challenge. TsuoA asthma [9, 14]. In patients with AlA, aspirin challenge
et al. [26] measured the production of LTB 4 and LTC4 in may lead to activation of peripheral blood platelets which
peripheral blood leucocytes stimulated by calcium iono- parallels the time course of bronchospastic reaction [36].
phore A 23187. They compared 4 groups (controls, In contrast to platelet activation, the detection of
AlA, atopic and intrinsic asthma) before and after endogenous platelet-activating factor (PAF) release has
indomethacin challenge. All three asthmatic groups not been a consistent finding. Aspirin-induced
produced more LTC4 than the healthy controls, but there bronchoconstriction does not seem to be based on the
was no difference between aspirin-intolerant patients and contracting properties of PAP [36].
atopic or intrinsic ones. LTB4 production (as well as PGE2 In 1982, MAcLOUF et al. [14) noticed that platelets
and TXB 2) was similar in all four groups. Indomethacin 12-HETE stimulated the generation of LTB 4 and
did not affect leukotriene generation in any of the groups 5-HPETE in mixed platelet-leucocyte suspension. These
studied. authors hypothesized that administration of aspirin to
The concept of arachidonic acid shunting needs an intolerant patients with asthma may lead to increased
additional assumption that the airways of aspirin- generation of 12-HPETE in their platelets [37] because
intolerant patients are more sensitive to leukotrienes than of impaired cyclooxygenase and 12-lipoxygenase balance,
those of other patients with asthma [27]. If not, all asth- or because of inhibition of peroxidase activity in
matic patients would react with bronchoconstriction in platelets. The released 12-HETE could activate 5-
response to aspirin-like drugs. Three research groups lipoxygenase of circulating blood leucocytes and pulmo-
addressed this problem. VAG!n et al. [28] and BIANco nary macrophages; generated leukotrienes would precipi-
[29] measured bronchial response to LTC4 in 10 aspirin- tate asthma.
sensitive asthmatics as compared to 10 controls. They AMEisEN and eo-workers [9, 10] reported that platelets
were unable to fmd any significant difference. SAKAKIBARA isolated from patients with aspirin-induced asthma react
et al. [30] studied airway responsiveness to methacho- abnormally in vitro to aspirin and other cyclooxygenase
line, histamine and LTD4 in 12 patients with AlA, 13 inhibitors by generating cytocidal molecules that can kill
patients with extrinsic asthma and 12 patients with parasitic larvae. Aspirin-like drugs had no similar effect
intrinsic asthma. There were no significant differences in on platelets from normal donors or allergic asthmatics.
either concentrations of any of the agents producing a This abnormality, according to the authors [38], appears
20% fall in forced expiratory volume in one second to be associated with the inhibiting properties of the
(FEV1) or the slope of FEV 1 changes among the groups analgesics on the cyclooxygenase pathway, that leads to
studied. The only positive finding was somewhat a defect of the binding of prostaglandin endoperoxide
delayed recovery in FEV 1 following challenge with PGI-Iz to its receptors on the platelet membrane.
LTD4 in the aspirin-intolerant group as compared to the NIZANKowsKA et al. [25] measured 12-HPETE produc-
others. These two studies do not support the concept of tion by platelets in 10 aspirin-sensitive asthmatics and 10
increased bronchial reactivity to LTC4 or LTD4 • How- matched healthy controls before and after administration
ever, the results of ARM et al. [3 1] suggest a selective of the threshold doses of aspirin. Initial levels of
increase in airway responsiveness of LTE4 • They meas- 12-HPETE did not differ between the two groups.
ured a 35% fall in the specific airway conductance Following aspirin challenge, 12-HPETE increase to
following histamine and LTE4 inhalation in 5 subjects similar levels in both groups. These data do not support
with aspirin-induced asthma and in 15 asthmatics with- a concept that there is a generalized abnormality
out aspirin sensitivity. The airways of aspirin-intolerant in arachidonic acid oxidative pathways in platelets of
patients had a significant, 13-fold increase in responsive- aspirin-sensitive asthmatics. Lack of protective effect of
ness to LTE4 relative to histamine when compared to prostacyclin infusions on aspirin challenge also raises
control asthmatics. Interestingly, this hyperresponsive- doubts about participation of platelets in the reactions
ness to LTE4 was abolished after aspirin-desensitization. discussed [33].
The concept of diversion of arachidonic acid
metabolism from prostanoids to leukotrienes is hard to
accept in view of likely compartmentalization of Compartmentalization of eicosanoids in the lungs
arachidonic acid in the lung [32]. This concept still awaits
testing with a powerful, specific leukotriene inhibitor. An interesting hypothesis was recently proposed by
In a recent trial [33] pretreatment of aspirin-intolerant GRYGLEWSKI [39]. It is based on the idea that arachidonic
asthmatics with leukotriene inhibitor failed to prevent acid metabolism in the lungs is compartmentalized [32].
aspirin-precipitated bronchospasm. The bioavailability of Thus, PGE2 is generated by smooth muscle of largeASPIRIN-INDUCED ASTHMA 591
airways, TXA 2 by contractile elements of lung numbers in response to viral infections and are highly
parenchyma, prostacyclin is produced by vascular specific.
endotheliwn, while leukotrienes are released by leucocytes 4) Lung macrophages produce PGE2 which suppresses
residing in the lungs and by fibroblasts. All these com- immunological response [49]. including cytotoxic activ-
partments can be stimulated simultaneously by an immu- ity of lymphocytes [50, 51]. This inhibition can be
nological reaction. overcome by anti-cyclooxygenase analgesics, which
The hypothesis postulates that in patients with deprive macrophages of PGE2 •
AlA cyclooxygenase of large airways is more Several of the hypotheses presented here are now being
susceptible to pharmacological inhibition than that of lung actively tested. New hypotheses might be expected to
parenchyma. In consequence, an ingestion of anti- emerge as the role of other eicosanoids, such as 15-
cyclooxygenase drug causes an increase in TXAjPGE 2 lipoxygenase products, become more clear. Though
ratio. The most likely explanation for an augmented aspirin-induced asthma guards its secrets well, it attracts
selective susceptibility of bronchial cyclooxygenase to more and more scientists and clinicians, convinced that
analgesics is chronic viral infection of the upper airways unravelling the mysteries of this syndrome will give new
of patients with AlA [40, 41]. This chronic infection insight into the pathogenesis of asthma.
might either change the biochemical characteristics of
cyclooxygenase in upper airways or make it easily
accessible to analgesics. If the hypothesis is right, then References
simultaneous pretreatment of patients with TXA 2 1. Samter M, Beers RF Jr. - Intolerance to aspirin. Clinical
synthetase inhibitor and TXAjPGHz receptor antagonist studies and consideration of its pathogenesis. Ann /nJ Med,
should protect them against the aspirin-induced 1968, 68, 975-983.
bronchoconstriction. 2. Virchow C. - Analgetika-Intoleranz bei Asthmatikem
(Analgetika-Asthma-Syndrom) vor laufige Mittelung. Prax
PneUJ7U)/, 1976, 30, 684-692.
Viral infection 3. Szczeklik A. - Analgesics, allergy and asthma. Drugs,
1986, 32, (Suppl. 4), 148- 163.
4. Schlumberger HD. - Drug-induced pseudo-allergic
Viruses have been implicated in pathogenesis of asthma syndrome as exemplified by acetylsalicylic acid intolerance.
[42, 43], including aspirin intolerance [4, 40], though in In: Pseudo-allergic reactions. Involvement of drugs and chemi-
the latter case no explanation was offered as to how viral cals. P. Dukor, P. Kallos, H.D. Schlumberger, G.B. West eds,
infection could be linked with cyclooxygenase- Karger, Base!, 1980, pp. 125-203.
dependent mechanism. Such explanation has been given 5. Szczeklik A, Gryglewski RJ, Czerniawska-Mysik G. -
by a recent hypothesis [41] . Relationship of inhibition of prostaglandin biosynthesis by
The hypothesis postulates that aspirin-induced asthma analgesics to asthma attacks in aspirin-sensitive patients. Br
results from chronic viral infection. In response to a virus, Med J, 1975, 1, 67-69.
a long time after the initial exposure, specific cytotoxic 6. Vane RI. - Inhibition of prostaglandin synthesis as a
lymphocytes are produced. Their activity is suppressed mechanism of action for aspirin-like drugs. Nature, 1971, 231,
232-234.
by PGE2 produced by pulmonary alveolar macrophages. 7. Szczeklik A, Gryglewski RJ, Czerniawska-Mysik G. -
Anti-cyclooxygenase analgesics block PGE2 production, Clinical patterns of hypersensitivity to nonsteroidal anti-
and allow cytotoxic lymphocytes to attack and kill their inflanunatory drugs and their pathogenesis. J Allergy Clin lm-
target cells, i.e. virus-infected cells of the respiratory tract munol, 1977, 60, 276-284.
During this reaction, toxic oxygen intermediates, 8. Stevenson DD, Lewis RA. - Proposed mechanisms of
lysosomal enzymes and mediators are released, which aspirin sensitivity reactions. J Allergy Clin Immunol, 1987, 80,
precipitate attacks of asthma. These acute attacks can 788-790.
be prevented by avoidance of all drugs with anti- 9. Ameisen JC, Capron A, Joseph M, Tonne) AB. -Platelets
cyclooxygenase activity. However, asthma continues to and aspirin-induced asthma. In: Clinical pharmacology and
therapeutic progress in asthma. A.B. Kay ed., Blackwell,
run a protracted course because of chronic viral London, 1986, pp 226-236.
infection. 10. Capron A, Ameisen JC, Joseph M, Auriault C,
The hypothesis is bases on the following concepts: Tonne) AB, Ca.en J. - New function for platelets and their
1) The clinical course of aspirin-induced asthma is pathological implications. 1nl Arch Allergy Appl Jmmunol, 1985,
reminiscent of viral infection [44, 45]. 77, 107-114.
2) Latency or semi-latency of viruses is being increas- 11. Szczeklik A, Nizankowska E, Dworski R, Splawinski J,
ingly recognized [46, 47]. In man, a notable example is Gajewski P, Splawinska B. - Effects of inhibition of throm-
Epstein-Barr virus, causing infection which persists for boxane A, synthesis in aspirin-induced asthma. J Allergy Clin
life, and is subject to reactivations. Interestingly, some of lmmunol, 1987, 80, 839-843.
the clinical manifestations of acute Epstein-Barr virus 12. Toogood JH. - Aspirin intolerance, asthma, prostaglandins
infection, such as Guillain-Barre syndrome, hepatitis or and cromolyn sodium. Chest, 1977, 72, 35-37.
13. Szczeklik A, Nizankowska E. Nizankowski R. -
suppression of haematopoiesis may be caused by secon- Bronchial reactivity to prostaglandins F. alpha, E, and hista-
dary immune responses to latently infected lymphocytes mine in different types of asthma. Respiration, 1977, 34,
[48]. 323-331.
3) Cytotoxic T-lymphocytes form a part of the human 14. Maclouf F, Fruteau de Laclos B, Borgeat P.- Stimulation
immune system in the respiratory tract. They increase in of leukotriene biosynthesis in human blood leukocytes by592 A. SZCZEKLIK platelet-derived 12-hydroperoxyeicosatetraenoic acid. Proc NaJI 31. Arm JP, O'Hickey SP, Spur BW, Lee TH. - Airways Acad Sci USA, 1982, 79, 6042-6046. responsiveness to histamine and lcukotriene E4 in aspirin- 15. Kuehl FA, Dougherty HW, Ham EA. - Interactions induced asthma. J Allergy Clin lmmunol, 1989, 83, 187, abstr. between prostaglandins and leukotrienes. Biochem Pho.rmacol, 187. 1984. 33, 1-5. 32. Gryglewski RJ, Dembinska-Kiec A, Grodzinska L, 16. Lee TH, Drazen JM, Leitch AG, Ravalese J, Corey Panczenko B. - Differential generation of substances EJ, Robinson DR, Lewis RA, Austen KF. - Enhancement with prostaglandin-like and thromboxane-like activities of plasma levels of biologically active leukotriene B by guinea-pig trachea and lung strips. In: Lung Cells in compounds during anaphylaxis of guinea-pig pretreated by Disease. E Bouhuys ed., Elsevier, Amsterdam, 1976, pp. indomethacin or a fish-oil enriched diet. J /mmunol, 1986, 136, 289- 307. 2575-2582. 33. Nizankowska E, Sheridan AQ, Maile MH, Cross CJ, 17. Dworski R. Shellcr JR, Wickersham NE, Oates JE, Brigham Czemiawska-Mysik G, Szczeklik A. - Pharmacological KL, Roberts U, FitzGerald GA. - Allergen-stimulated release attempts to modulate leukotriene synthesis in aspirin-induced of m ediators into sheep bronchoalveolar lavage fluid. Effect of asthma. Agents Actions Supp/, 1987, 21, 203- 213. cyclooxygenase inhibition. Am Rev Respir Dis, 1989, 139, 34. Morley J, Page CP, Sanjar C. - Platelets in asthma. 46-51. Lancet, 1985, ii, 726-727. 18. Undem BI, Piclett WC, Lichtenstein LM. Adams II OK. 35. Szczeklik A, Milner PC, Birch J, Watkins J, Martin JP. - - The effect of indomethacin on immunologic release of Prolonged bleeding time, reduced platelet aggregation, histamine and sulfidopeptide leukotrienes from human altered P AF-acether sensitivity and increased platelet mass are bronchus and lung parenchyma. Am Rev Respir Dis, 1987, 136, a trait of asthma and hay fever. Throm Haemost, 1986, 56, 1183- 1187. 283-287. 19. Vigano T, Toia A, Crivellari MT, Galli G, Mezzetti M, 36. Schmitz-Schumann M, Menz G, Schaufele A, von Felten Folco GC. - Prostaglandin synthetase inhibition and formation A, Matthys H, de Souza V, Virchow C.- Evidence of PAP of lipoxygenase products in immunologically challenged nor- release and platelet activation in analgesics-asthma-syndrome. mal human lung parenchyma. Eicosanoids, 1988, 1, 73- 77. Agents Actions Suppl, 1987, 21, 215- 224. 20. Ortolani C, Mirone C, Fontana A, Folco GC, Miadonna 37. Borgeat P, Fruteau de Laclos B. Maclouf J.- New con- A, Montalbetti N, Rinaldi M, Sala A, Tedeschi A, Valente D. cepts in the modulation of leukotriene synthesis. Biochem - Study of mediators of anaphylaxis in nasal wash fluids after Pharmacol, 1983, 32, 381- 387. aspirin and sodium metabisulfite nasal provocation in intoler- 38. Joseph M, Capron A, Ameisen JC, Martinot JB, Tonne! ant rhinitic patients. Ann Allergy, 1987, 59, 106- 112. AB.- Plaquettes sanguines et asthme a l'aspirine. Al/ergie lm- 21. Ferreri NR. Howland WC, Stevenson DD, Spielberg HL. munol, 1987, 19 (Suppl.), 7- 10. - Release of leukotrienes, prostaglandins and histamine into 39. Gryglewski RJ. - Eicosanoids in aspirin-induced asthma. nasal secretions of aspirin-sensitive asthmatics during reaction Agents Actions Supp/, 1989, 28, 113-121. to aspirin. Am Rev Respir Dis, 1988, 137, 847-854. 40. Gryglewski RJ, Szczeklik A, Nizank:owska E. - Aspirin- 22. Bisgaard H, Robinson C, Roemling F, Mygind N, Church senstive asthma: its relationship to inhibition of prostaglandin M, Holgate S. - Leukotriene c. and histamine in early allergic biosynthesis. In: Prostaglandins and Thromboxanes. F. Berti, reaction in the nose. Allergy, 1988, 43, 219-227. B. Samuelsson, G .P. Velo eds, NATO Advanced Study Insti- 23. Godard P, Chaintreuil J, Damon F, Coupe M, Aandre 0, tute, Series A, Life Sci, Plenum Press, New York, 1977, pp. Crastes de Paulet A, Michel FB. - Functional assessment of 566-570. alveolar macrophages: comparison of cells from asthmatic and 41. Szczeklik A.- Aspirin induced asthma as a viral disease. normal subjects. J Allergy Clin lmmunol, 1982, 70, 88-93. Clin Allergy, 1988, 18, 15-20. 24. Goetzl EJ, Valacer DJ, Payan DO, Wong MYS.- Abnor- 42. Welliver RC, Ogra PL. - Immunology of respiratory viral mal responses to aspirin of leukocyte oxygenation of arachidonic infections. Ann Rev Med, 1988, 39, 147-162. acid in adults with aspirin intolerance. J Allergy Clin lmmunol, 43. Prick WE, Busse WW. - Respiratory infections: their role 1986, 77, 693~98. in airway responsiveness and pathogenesis of asthma. Clinics 25. Nizankowska E, Michalska Z, Wandzilak M. Radomski Chest, 1988, 9. 539- 549. M, Marcinkiewicz E. Gryglewski RJ, Szczeklik A. - An 44. Szczeklik A. - Origin of aspirin-induced asthma. Agents abnormality of arachidonic acid metabolism is not a general- Actions Supp/, 1989, 28, 27-39. ized phenomenon in patients with aspirin-induced asthma. 45. Prieto L, Palop J, Castro J, Basomba A. - Aspirin-induced Eicosanoids, 1988, 1, 45-48. asthma in a patient with asthma previously improved by non- 26. Tsuda M , Sakakibara H. Kamidaira T, Saga T, Suetsugu steroidal antiinflarnmatory drugs. Clin Allergy, 1988, 18, S, Umeda H. - Arachidonic acid metabolism of peripheral 629- 632. blood leukocytes in aspirin-induced asthma. N Engl Region 46. Podgwaite ID, Mazzone HM. - Latency of insect virus. Allergy Proc, 1988, 9, 437, abstr. 755. Adv Virus Res, 1986, 31, 293-317. 27. Szczeklik A, Gryglewski RI. - Asthma and antiinflarnma- 47. Anonymous.- Herpes simplex virus latency. Lancet, 1989, tory drugs. Mechanisms and clinical patterns. Drugs, 1983, 25, 1, 194-195. 533-543. 48. Pagano JS. - Epstein-Barr virus. In: Anti-viral drug devel- 28. Vaghi A, Robuschi M, Simone P, Bianco S. - Bronchial opment. A multi-disciplinary approach. E De Clerq, RT Walker, response to leukotriene c. (LTC.) in aspirin asthma. Abstracts eds, NATO Advanced Study Institutes Series A, Life Sci, SEP 4th Congress, Milano-Stresa, 1985, 171. Plenum Press, New York, 1988, 143, 81-90. 29. Bianco S. - Asthme et medicaments anti-inflarnmatoires 49. Morley J. - Prostaglandins and lymphokines in arthritis. non-steroidiens. In: Allergologie. J. Charpin ed., Aarnmarion. Prostag/andins, 1974, 8, 315-326. Paris, 1986, p. 683. 50. Herberman RB. - Natural killer cells in lungs and other 30. Sakakibara H, Suetsugu S, Saga T, Handa M, Suzuki M, tissues and regulation of their activity by suppressor cells. J Doizoe T, Minako T, Horiguchi T, Konishi Y, Umeda H. - Allergy Clin /mmunol, 1986, 78, 566-570. Bronchial hyperresponsiveness in aspirin-induced asthma. J 51. Roitt IM, Brostoff J, Male D. - lnununology. Churchill lap Thor Soc. 1988, 26, 612-619. Livingstone, London. 1985.
ASPIRIN-INDUCED ASTIIMA 593 La theorie de la cyclooxygenase pour /'asthma induit par 1~ fois expe(imerttale.S et ciWqucs. Blle a sclmule'\m certain l'Aspirine. A. Szczeklik. nombre d'hypotheses . ·~ur. lcs mecanis~es ,de, la btonchocon- RESUME: L'aslhmc induit par I'Aspirine est un syndrome strictiQn. ;routes ces hypotheses dkrites. ici se developpment clinique bien defini, qui atteint environ 10% des aslhmatiques dans le caare de ia. tMorle d~ la pycJooi!,ygenase. Leur suppo- adultcs. Chcz ces patients, l'Aspirine et d'auttes analg~siques sition pPhcip8.J.e est q\Je l'inhlbiti~ri de la cyclooxygenase pouvent declencher des crises asthmatiques. L'idee que la crise stimUJ.~ des· reactionS biochirniques spkifiques que conduisent pourrait r~sulter de l'inhibition sp&:ifiquc d'une scule enzyme, ~des crises ·d'astllma frlil).c• .. . ,. en !'occurrence la cyclooxygenase, ttouve des confirmations ~ Eru Reipfr J.; 1990, 3, 587-S93.
You can also read