Review of high-dose intravenous vitamin C as an anticancer agent
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
bs_bs_banner
Asia-Pacific Journal of Clinical Oncology 2014; 10: 22–37 doi: 10.1111/ajco.12173
REVIEW ARTICLE
Review of high-dose intravenous vitamin C as
an anticancer agent
Michelle K WILSON,1* Bruce C BAGULEY,2 Clare WALL,2 Michael B JAMESON3 and
Michael P FINDLAY2
1
Auckland City Hospital, 2University of Auckland, Auckland and 3Waikato Hospital, Hamilton, New Zealand
Abstract
In the 1970s, Pauling and Cameron reported increased survival of patients with advanced cancer treated
with high-dose intravenous (IV) vitamin C (L-ascorbate, ascorbic acid). These studies were criticized for
their retrospective nature and lack of standardization of key prognostic factors including performance
status. Subsequently, several well-designed randomized controlled trials failed to demonstrate a significant
survival benefit, although these trials used high-dose oral vitamin C. Marked differences are now recog-
nized in the pharmacokinetics of vitamin C with oral and IV administration, opening the issue of thera-
peutic efficacy to question. In vitro evidence suggests that vitamin C functions at low concentrations as
an antioxidant but may have pro-oxidant activity at high concentrations. The mechanism of its pro-
oxidant action is not fully understood, and both intra- and extracellular mechanisms that generate hydro-
gen peroxide have been proposed. It remains to be proven whether vitamin C-induced reactive oxygen
species occur in vivo and, if so, whether this will translate to a clinical benefit. Current clinical evidence
for a therapeutic effect of high-dose IV vitamin C is ambiguous, being based on case series. The inter-
pretation and validation of these studies is hindered by limited correlation of plasma vitamin C concen-
trations with response. The methodology exists to determine if there is a role for high-dose IV vitamin C
in the treatment of cancer, but the limited understanding of its pharmacodynamic properties makes this
challenging. Currently, the use of high-dose IV vitamin C cannot be recommended outside of a clinical
trial.
Key words: cancer, pro-oxidant, vitamin C.
INTRODUCTION 1950s, vitamin C was originally hypothesized to be pro-
tective against cancer,1,2 but in the 1970s, Ewan Cameron
Vitamin C is an essential micronutrient for humans, who and Linus Pauling suggested that it also had a therapeutic
lack the enzyme required for its synthesis. Vitamin C is effect, reporting increased survival of patients with
well known for its antioxidant activity although it is only advanced cancer following high-dose IV vitamin C treat-
one of a large variety of dietary antioxidants. In the ment.3 In contrast, several subsequent randomized con-
trolled trials (RCTs) of high-dose oral vitamin C failed to
demonstrate a similar benefits,4–6 opening the issue of
Correspondence: Dr Michelle K Wilson MBChB, Auckland
City Hospital, 85 Park Road, Grafton, Private Bag 92019,
therapeutic effectiveness to controversy.
Auckland 1023, New Zealand. More recent studies showed that high plasma concen-
Email: michelle.wilson@uhn.ca trations of vitamin C can only be achieved if vitamin C
*Present address: Princess Margaret Hospital, Toronto, is administered intravenously or intraperitoneally as the
Canada. rate of absorption from the gut is limited with oral
Conflict of interest: none administration.7,8 Plasma concentrations in humans fol-
Accepted for publication 25 November 2013. lowing high-dose IV vitamin C are approximately 200
© 2014 Wiley Publishing Asia Pty LtdHigh-dose IV vitamin C as anticancer agent 23
times higher than those achieved following oral admin- HOW DOES VITAMIN C POTENTIALLY
istration.8 High-dose oral and high-dose IV vitamin C EXERT ITS ANTITUMOR ACTIVITY?
treatment therefore have to be considered as distinct
therapeutic approaches. One of the issues with the use of high-dose IV vitamin C
The purpose of this review is to survey the literature is the lack of understanding of its potential mechanism of
to analyze the antitumor effects of vitamin C in both action. Although initial theories centered on modification
human and animal studies in terms of the dose and of biological responses, more recent research has concen-
achieved plasma vitamin C concentrations. In analyzing trated on the importance of both extracellular and intra-
the effects of vitamin C in rodent models, it must be cellular effects of vitamin C. McCormick postulated that
noted that rodents synthesize their own vitamin C and vitamin C protected against cancer by increasing collagen
therapeutic effects are limited to “high plasma vitamin synthesis.1 Cameron and Pauling later hypothesized that
C.” The five questions this review will address are there- the association between vitamin C and hyaluronidase
fore (i) Does vitamin C (at high plasma concentrations) activity was key. They speculated that a high intake of
have antitumor activity in rodent systems? (ii) If so, how vitamin C increased the biosynthesis of a physiological
does vitamin C potentially exert its antitumor activity? inhibitor of hyaluronidase (PHI) and subsequently
(iii) Does vitamin C (at high plasma concentrations) reduced the invasiveness of proliferative disease.13
have clinical antitumor activity? (iv) Is vitamin C safe Experimental studies, while demonstrating that scorbutic
alone and in conjunction with chemotherapy? and (v) If guinea pigs have higher levels of PHI compared with
the answer is not clear, how can the issue of therapeutic those receiving oral vitamin C, failed to show a link
efficacy be addressed in the future? between PHI levels and the amount of vitamin C given.14
High-dose oral vitamin C has been shown to be a
potent immunomodulator, enhancing the activity of
DOES VITAMIN C (AT HIGH PLASMA natural killer (NK) cells in vivo.15 NK cells are thought
to be important in immune surveillance, preventing
CONCENTRATIONS) HAVE
growth and dissemination of tumor cells.16 Heuser and
ANTITUMOR ACTIVITY IN Vojdani demonstrated that vitamin C caused an increase
RODENT SYSTEMS? not only in NK activity but also B- and T-cell activity in
Despite a large amount of preclinical in vitro data on the patients previously exposed to a toxic chemical.17 Both
effects of vitamin C on tumor cells, there are few reports these studies used oral vitamin C. Other studies have
on the antitumor activity of high-dose vitamin C in found evidence to the contrary so while it is a potential
xenografts of human tumors in immunodeficient mice, a mechanism, this remains unproven.18
well-characterized method of predicting potential anti-
tumor activity in humans (Appendix I). Chen et al. dem- Extracellular mechanisms for
onstrated that vitamin C (4 g/kg intraperitoneal [IP] vitamin C action
once or twice daily) reduced tumor growth and weight Studies by Chen et al. established that high doses of
by 41–53% using ovarian (Ovcar5), pancreatic vitamin C in mice (IV or IP) generated significant extra-
(PAN01) and glioblastoma (9 L) tumor cell lines in cellular concentrations of hydrogen peroxide (H202).19
mice.9 The effective plasma concentration that decreased H202 is central to a diverse range of physiological
survival by 50% (EC50) was less than 10 mM in 75% of responses pivotal to disease progression, including
the tumor cells tested, but in contrast cytotoxicity was angiogenesis, and oxidative stress.20 They hypothesized
not evident in normal cells at ascorbate concentrations that the presence of a catalyst such as ferric ion within
exceeding 20 mM. The lack of any complete response the extracellular matrix of tumors could oxidize vitamin
led them to propose that the role for vitamin C may be C to ascorbate radicals, which then could then donate
as an adjunct alongside chemotherapy. Similar results electrons to oxygen to form superoxide radicals (O2−)
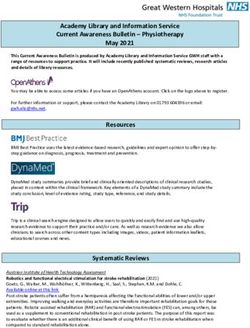
have been shown in mesothelioma cell lines.10 In keeping (Fig. 1). This would be converted by superoxide dis-
with this premise, Espey et al. demonstrated that mutase to the potentially tumoricidal peroxide ion.19
vitamin C (4 g/kg) inhibited the growth rate of PAN02 Because peroxide is rapidly converted to oxygen in
pancreatic tumors by approximately 40%, and also aug- blood, the accumulation of peroxide would occur only
mented the effect of concurrently administered gemcit- in tissues. The identity of the catalyst was not elucidated
abine (30 mg/kg).11 This dose of vitamin C in rodents is but a later study suggested that it could be the metallo-
equivalent to 1.5 g/kg in humans.12 protein ferritin, which is secreted by some tumor cells.22
Asia-Pac J Clin Oncol 2014; 10: 22–37 © 2014 Wiley Publishing Asia Pty Ltd24 MK Wilson et al.
The selective cancer cell death may be explained by the
Extracellular ascorbate
different mechanisms of ATP generation with cancer
cells primarily using anaerobic generation and normal
cells aerobic generation. It remains uncertain whether
Neutrophil
Metallo- this in vitro and in vivo data will translate into clinical
protein BH4 benefit.
complex
e¯
Intracellular mechanisms for
vitamin C action
NADPH oxidase
O2 O2˙¯ O2 O2˙¯ Vitamin C is taken into cells by sodium-dependent
vitamin C transporter 1 (SVCT1) and SVCT2 sodium-
Superoxide dependent transporters that are members of the SLC23
dismutase
family.24 The oxidized form of vitamin C, dehydroascor-
H2O2 H2O2
bate, can also be taken up by the glucose transporter
Figure 1 Extracellular mechanism of action of high-dose (GLUT) and reduced in the cell to ascorbate.25 Macro-
intravenous vitamin C. Legend: Two possible mechanisms for phages and vascular endothelial cells can express high
the generation of extracellular hydrogen peroxide (H2O2) in levels of SCVT2 and thus concentrate vitamin C to a
response to ascorbate. In the first, as shown on the left-hand
high millimolar degree.26,27
side, molecular oxygen is reduced to superoxide by a molecular
complex of ascorbate and an as yet uncharacterized metallo- High intracellular vitamin C concentrations are pro-
protein catalyst (such as ferritin).9 In the second, as shown on posed to inhibit hypoxia-inducible factor (HIF)-1α
the right-hand side, ascorbate is first taken up by a cell such as activation. HIF plays an important role in determining
a neutrophil, either directly by an ascorbate transporter or patterns of gene expression in cancer and is another
indirectly as dehydroascorbate by the glucose transporter. Here
potential target of vitamin C action. HIF-1α is broken
it stabilizes tetrahydrobiopterin (BH4), preventing its degrada-
tion and leading to activation of the enzyme nicotinamide down by hydroxylases, which require iron and ascorbic
adenine dinucleotide phosphate (NADPH) oxidase, which acid as cofactors.28 Vitamin C deficiency has been shown
reduces molecular oxygen to superoxide.21 In each case, per- in vitro to compromise hydroxylation of HIF and upregu-
oxide is generated subsequently by the enzyme superoxide late HIF-1α.29,30 HIF-1α overexpression promotes tumor
dismutase. progression through angiogenesis, confers resistance
to chemotherapy and radiotherapy, and carries a poor
prognosis.29,31,32
Extracellular peroxide, depending on concentration, can Vitamin C levels have been studied in patients with
have a cytotoxic effect. endometrial cancer, demonstrating significantly lower
Chen et al. demonstrated that high (pharmacologic) levels of vitamin C in high-grade tumors compared with
but not low (physiological) concentrations of vitamin C paired normal tissue.33 Markers of HIF-1 activation
killed cancer but not normal cells, with cell death depen- (HIF-1α protein, GLUT-I and BCL 2/adenovirus E1B)
dent on extracellular vitamin C concentrations.9,19,23 were elevated in samples with low vitamin C levels, in
H202 generation displayed a linear relationship with the keeping with the theory that low tissue vitamin C con-
formation of the vitamin C radical.23 The pattern of cell centrations upregulate the HIF-1 pathway. There was
death changed from apoptosis to pyknosis/necrosis as also an inverse correlation between vascular endothelial
vitamin C concentrations increased, in keeping with growth factor levels and vitamin C concentrations.
H202-mediated cell death.23 When H202 scavengers Similar findings have been demonstrated in gliomas.32
were employed they were protective against cell death.23 Genetic approaches and small-molecule inhibitors tar-
H202 generated by vitamin C oxidation and exog- geting HIF-1 have proven effective at decreasing resis-
enously added H202 produced cell death curves that tance to chemotherapeutics in a number of different
were indistinguishable.23 cancers.34,35 A study using vitamin C with a recombinant
Chen et al. did not demonstrate a lower level of anti- adenovirus-associated virus (rAAV) vector bearing
oxidant enzymes (catalase, superoxide dismutase and small-interfering RNA targeting HIF-1α (rAAV-siHIF)
glutathione peroxidase) in malignant cells to explain the in pancreatic tumors in athymic mice found that vitamin
selective death of cancer cells.23 Instead they theorized C could inhibit expression of HIF-1α protein but not
that H202 diffuses into sensitive cancer cells and causes messenger RNA expression.36 It inhibited the growth in
toxicity by adenosine triphosphate (ATP) depletion.19 early and middle stages of disease but not advanced
© 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37High-dose IV vitamin C as anticancer agent 25
stages. The lack of blood supply in advanced stages is have chronic pancreatitis on autopsy. A further patient
thought to compromise the delivery of vitamin C to the with lymphoma had evidence of remission while on
tumor.36 vitamin C, which recurred once treatment stopped.
Remission was again achieved on restarting vitamin C.38
DOES VITAMIN C (AT HIGH PLASMA Another 48% of patients reported a subjective improve-
CONCENTRATIONS) HAVE CLINICAL ment quantified by a reduction in analgesic use and need
for paracentesis.37 However, with no control group, a
ANTITUMOR ACTIVITY?
placebo effect for these symptomatic improvements
In 1974 Cameron and Campbell published the first clini- cannot be excluded.
cal trial suggesting the therapeutic role for vitamin C in Cameron and Pauling then published two historically
cancer.37 Fifty patients with no further conventional controlled trials, each comparing 100 patients treated
treatment options were treated with IV and oral vitamin with high-dose vitamin C, with 1000 controls matched
C (20% receiving oral only). Of five tumor regressions by age, sex, tumor site and histological features
described, one occurred in a patient with ovarian cancer (Fig. 2).3,39 Both trials found a significant prolongation
who had extensive pelvic disease at initial diagnostic of mean survival (210 vs 50 days and 293 vs 39 days,
laparotomy, which was not present at autopsy. respectively).3,39 However, neither of these trials was
However, this did not seem to prolong survival with the standardized by two critical prognostic factors: perfor-
patient dying on day 33. Two other patients reported to mance status and stage.4,40 The consistency of determi-
have regression had no histological diagnoses of incur- nation of “untreatability” is also controversial in the
able disease. One of these patients diagnosed with initial trial: 20% of the control group died within a few
advanced pancreatic cancer at laparotomy was found to days of being deemed untreatable compared with none
400
350
300
Average survival (days)
250
200
150
100
50
0
Cameron Cameron Murata, Murata, Cameron Creagan Tschetter Moertel
and Pauling and Pauling Mortshige Mortshige and 1979 ++ ^ 1983 ++ ^ 1985 ^
1976 + 1978 + 1982 * 1982 ++ ** Campbell
(Kamioka (Fukuoka) 1991 +
Kozan)
Figure 2 Clinical studies of high-dose vitamin C in cancer. Summary of survival results from published studies on high-dose
vitamin C administration to patients with cancer. Control patients are shown in yellow and patients treated with vitamin C in blue.
The bars on the left-hand side represent trials with intravenous administration and those on the right with oral administration.
+Reported mean survival. ++Reported median survival. ∧Randomized controlled trials. *Compared nil versus low versus high-dose
vitamin C using combination of oral and intravenous (IV) dosing. **Compared low versus high-dose vitamin C using combination
of oral and IV dosing.
Asia-Pac J Clin Oncol 2014; 10: 22–37 © 2014 Wiley Publishing Asia Pty Ltd26 MK Wilson et al. in the treatment group. The latter trial retrospectively Since this time case series and reports have continued analyzed the time from first hospital admission to date to raise interest in a therapeutic role for high-dose of untreatability (>1 year in 27% of patients in the Vitamin C, but there has been limited correlation with treatment group and 23% in control group – not statis- plasma vitamin C concentrations.38,45–49 Riordan et al. tically significant) to address this concern. and Padayatty et al. published the largest of these series The Mayo Clinic conducted three RCTs to examine with seven45 and three patients, respectively.46 These the efficacy of vitamin C (Fig. 2), none of which showed series cover a spectrum of malignancies but there is a definitive benefit in terms of survival or quality of significant overlap, with many of these cases published life.4–6 They compared 10 g of vitamin C administered repeatedly.37,38,45–47 Padayatty et al. reported on three orally versus placebo. The initial trial used patients patients with renal cell cancer (RCC), non-Hodgkin unsuitable for further systemic therapy either because of lymphoma (NHL) and bladder cancer, in keeping with progression during treatment or because their general the guidelines of the US National Cancer Institute Best condition precluded further treatment.4 These negative Case Series Program.46 Two of these patients were also results were refuted due to concerns that, if vitamin C included in the Riordan series et al.45 acts by improving host resistance, prior treatment would All of these patients used IV vitamin C either with obscure any benefit.41,42 All but nine patients had had standard therapy or alongside other alternative thera- previous treatment compared with only 4 of the 100 pies, making it impossible to definitively assign clinical patients in the initial Cameron and Pauling trial.42 benefit to vitamin C. They described positive results The second RCT was conducted in 100 cytotoxic- with either improved health status or slowed disease naive patients with colorectal cancer.5 None of the 38 progression but with no control group this is inconclu- patients with measurable disease demonstrated disease sive. RCC is a malignancy that has a variable natural response.5 This trial was criticized as it was limited to history and one that rarely can undergo spontaneous patients with colorectal cancer and it was questioned regression (although usually following nephrectomy, whether negative results in this tumor group were which was not the case in this report).50 The latter two transferable to other primary sites.5,40 However, 20% of cases reported received standard therapy with radiation the patients in the initial Cameron and Pauling trial and surgery, respectively. All of these cases had the slim had colorectal cancer and they demonstrated similar potential for long-term remission with these therapies.51 survival benefit to other tumor subtypes.3,39 Subse- Interestingly, the case of NHL did have nodal relapse quently, 144 patients with predominantly lung and confirmed histologically that appeared to regress with colorectal primaries were studied. They described an vitamin C. There were no plasma vitamin C concentra- initial benefit in overall well-being but this was lost by tions measured to help establish a dose–response rela- 6 weeks.6 tionship. There was often a lack of histological diagnosis The results from these RCTs led to the current in the case of recurrent or metastatic disease. In view of opinion among oncologists that high-dose vitamin C is these factors, they do not provide definitive evidence of ineffective. However, it is now recognized that vitamin C a beneficial or detrimental role for IV vitamin C. pharmacokinetics differ significantly with oral and IV Drisko et al. published a case series of two patients dosing.8 Plasma vitamin C concentrations were not mea- with advanced stage IIIc ovarian cancer treated with sured in any of these trials. chemotherapy and IV vitamin C (60 g twice weekly).49 Around this time two further trials published similar Both these patients had optimal surgical debulking, a results to the historical trials, demonstrating prolonga- key prognostic determinant of patient outcome.52 These tion of survival times and improvement in quality of life patients were reported to demonstrate prolonged sur- (Fig. 2).43,44 Cameron and Campbell published the only vival with both patients alive over 3 years out from trial that measured plasma levels in association with diagnosis. Treated stage IIIc ovarian cancer has a 5-year survival time and demonstrated a linear relationship survival rate of around 30%.53 The survival for these between dose and IV plasma vitamin C levels.43 Levels patients consequently may be explained by optimal con- above 3 mg/dL (0.17 mM) were reported as desirable ventional therapy. However, neither of these patients but this concentration based on recent literature appears had subsequent chemotherapy over this time. too low to exert a pro-oxidant effect.43 Treatment was A recent retrospective multicenter epidemiological not randomized and was dependent on clinician prefer- cohort study examined the effect of IV vitamin C (7.5 g ence, creating the potential for selection bias. Again weekly) on quality of life during adjuvant chemotherapy there was no stratification by performance status. and radiotherapy and aftercare in patients with breast © 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37
High-dose IV vitamin C as anticancer agent 27
cancer.54 Mean intensity scores in patients treated with but not 30 g was effective at attaining this. A recent
vitamin C during adjuvant therapy were improved (0.25 phase I trial of 24 patients demonstrated that IV vitamin
vs 0.4, P = 0.013), but the absolute difference was small C to a dose level of 1.5 g/kg three times per week was
and unlikely to be of clinical significance (score of 0 safe and achieved plasma concentrations >10 mM for
representing no symptoms and 1 representing mild com- several hours.58 While average follow-up was only 10
plaints). They showed a mean Eastern Cooperative weeks, this trial did not demonstrate objective tumor
Oncology Group performance status during adjuvant response at these levels.58 Riordan et al. published a
therapy of 1.596 in the study group and 2.067 in the series of 24 patients treated with IV vitamin C 150 mg/
control (P = 0.002). Tumor status was reported to be kg/day and 710 mg/kg/day.59 The mean plasma level was
stable at 6 and 12 months; however, longer follow-up is 1.1 mM (below the expected therapeutic target). They
necessary to evaluate the effect on survival and relapse, did not demonstrate a correlation between dose and
two critical outcomes of adjuvant therapy. plasma concentration. The reason for this is unclear.
In palliative patients, improvement in quality of life is Other factors such as critical illness, renal function and
an important component of care and a key end point.55 chemotherapy regimens may alter plasma vitamin C
Yeom et al. investigated the effect of IV vitamin C (10 g concentrations and affect the plasma concentration nec-
twice a day for 3 days) on quality of life in 39 palliative essary for cytotoxicity.58
patients.56 They demonstrated a significant improve-
ment in quality of life with higher scores for physical,
IS VITAMIN C SAFE ALONE AND IN
emotional and cognitive function and lower scores for
fatigue, nausea and vomiting, pain and appetite loss
CONJUNCTION WITH CHEMOTHERAPY
(both P < 0.005) in a single assessment 1 week posttreat- AND RADIOTHERAPY?
ment.56 Although these results are suggestive of a Vitamin C is generally regarded as an innocuous com-
benefit, with no control group a placebo effect cannot be pound with a favorable therapeutic index (Table 1).
excluded. The duration of benefit was also not assessed. While many centers have used high doses, there is
Cameron hypothesized palliative patients experience limited published data around the safety at these doses.
a strong “reverse placebo effect” because of previous Cases of acute hemolysis in patients with underlying
treatment failures that counterbalance “placebo and glucose-6-phosphate dehydrogenase (G6PD) deficiency
anticipation” effects.3,39 This has not been described in have been reported in patients treated with high-dose
the literature elsewhere.
Clinical pharmacokinetics
Table 1 Side effects of intravenous vitamin C
Vitamin C has different functions at physiological and
Major side effects
pharmacological plasma concentrations. Oral vitamin C
Glucose-6-phosphate deficiency†
administration is associated with tightly controlled Renal stones – particularly oxalate stones†
plasma concentrations regulated by the pharmacoki- Tumor acceleration
netic principles of bioavailability and clearance.7 Satu- Minor side effects
ration of bioavailability mechanisms occurs at oral Dyspepsia, nausea and altered bowel habit
doses of 400 mg daily equating to blood levels of Increase iron absorption‡
60–100 μM.8 IV dosing bypasses this tight control, Raise urinary uric acid levels and excretion of calcium and
achieving plasma concentrations up to 20 mM.7,8 iron60
In vitro evidence suggests plasma concentrations of Fluid overload – caution in patients with ascites, heart
10 mM are necessary for an antitumor effect and this failure
Interfere with routine laboratory parameters – B12, glucose
appears achievable clinically only with IV administra-
and fecal occult blood
tion.8 Padayatty et al. postulated that the vitamin C-free
Side effects falsely attributed to vitamin C60,61
radical species, ascorbyl radical, forms only when Mutagenicity
human plasma concentrations are greater than 10 mM Rebound scurvy
and that it is this radical or its unpaired electron that Infertility
induces oxidative damage in cancer cells.8 Hypoglycemia
Casciari et al. published a trial to determine the dose Destruction of vitamin B12
necessary to achieve this level in humans.57 In a single †
Absolute contraindication to intravenous vitamin C. ‡Important in
patient with colon cancer, they found that a dose of 60 g patients with hemochromatosis.
Asia-Pac J Clin Oncol 2014; 10: 22–37 © 2014 Wiley Publishing Asia Pty Ltd28 MK Wilson et al.
vitamin C with at least one fatality described.62,63 All 5-fluorouracil (5FU), sodium d-ascorbate and radia-
patients should be screened for G6PD deficiency prior to tion.75 Contrary to this, Witenberg et al. demonstrated a
starting vitamin C therapy.62 reduction in apoptosis from ionizing radiation in myeloid
Caution should also be taken in patients with a leukemia cells treated with dehydroascorbic acid.76
history of renal stones.60 Acute obstructive renal failure This sensitizing effect is postulated to be due to the
secondary to oxalate stones has been reported in a increased H202 generation secondary to vitamin C
patient with underlying renal impairment.64 The effect of administration. However, vitamin C may also result in
vitamin C on oxalate excretion is controversial with a reduction in HIF-1, which in xenograft models has
some believing excessive ingestion of AA increases the been shown to be associated with heightened radiation
formation of oxalate stones.60 sensitivity.77 In keeping with this theory, putative
In patients with widespread and rapidly proliferating small-molecule inhibitors of HIF-1 have demonstrated
tumors, vitamin C has been reported to cause tumor enhanced tumor responsiveness to radiation in vitro,
acceleration and precipitate tumor hemorrhage and supporting the use of this as a target in association with
necrosis.37,65 The initial Cameron and Campbell trial conventional therapies.78
described four patients in this category.37 Potentially, this High-dose vitamin C is also postulated to reduce the
could also be explained by the natural history of the toxicity of chemotherapy because of restoration of
underlying cancer. plasma vitamin C concentrations and thus antioxidant
Dyspepsia, nausea and altered bowel habit are the capacity.54 In vitro evidence has also suggested that
most frequently reported side effects, particularly fol- vitamin C may reduce the cardiac toxicity associated
lowing oral administration.59,60 High doses of oral with doxorubicin without compromising efficacy, pos-
vitamin C have been shown to affect iron absorption tulated to be related to peroxidation of cardiac lipids.79
and interfere with many routine laboratory param-
eters.59,60 In patients with congestive heart failure and HOW CAN THE ISSUE OF
ascites, the high fluid intake associated with administra- THERAPEUTIC EFFICACY BE
tion may exacerbate their condition.60
ADDRESSED IN THE FUTURE?
Role of vitamin C in combination with There are a number of trials underway in both the phase
chemotherapy and radiation I and II setting (Table 2). Although the methodology
The literature reports that 30–95% of patients with exists for investigating the role of high-dose vitamin C in
cancer try unconventional therapies, with the majority cancer therapy, it is hindered by uncertainties including
using these as adjuncts to their standard care with the the target population and markers and predictors of
intention to improve their quality of life and symptom response. The inconsistency and current level of evi-
control.66–68 Despite this wide use, it remains unclear dence to support a clear scientific rationale also makes
whether the concurrent use of antioxidants with chemo- the likelihood of funding for the necessary research
therapy and radiotherapy is beneficial or detrimental.69 problematic.
Because of the paucity of clinical trial evaluation, the The ready availability and accessibility of IV vitamin
evidence to date is mostly derived from in vitro and in C to patients makes a true placebo-controlled trial dif-
vivo data, and observational records. There are no pub- ficult, with crossover likely to be a major confounding
lished RCTs examining high-dose IV vitamin C in con- factor. Comparison of high-dose oral versus high-dose
junction with chemotherapy or radiotherapy, making it IV vitamin C (at least 1.5 g/kg three times per week as
difficult to definitively assess safety and efficacy. used in phase I trials) may address this, that is, low
Vitamin C has been studied in combination with a plasma concentration versus high (Table 3). A third
number of cytotoxic agents in vitro and in vivo with placebo-controlled arm could be added.
conflicting outcomes on efficacy11,70–72 (see Appendix II). The target population remains unclear. There is no
It is theorized that vitamin C may sensitize refractory clear cancer type or phase of care defined for the role of
cancers to radiotherapy and chemotherapy.11,70–73 Koch vitamin C. In view of this, vitamin C could be trialed as an
and Biaglow studied dehydroascorbic acid alongside isolated treatment in patients who have exhausted con-
radiation in hypoxic Ehrlich cells in ascites in vivo, ventional treatments. An alternative approach would be
demonstrating increased inhibition of cell growth with to use vitamin C in patients on observation such as those
half the radiation dose.74 Similar findings were found in with asymptomatic biochemical progression of ovarian
neuroblastoma and glioma cell lines treated with or prostate cancer, asymptomatic pulmonary metastases
© 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37Table 2 Current clinical trials
Investigator Phase Title Dose of vitamin C Objectives
Levin I A phase I study of high-dose IV vitamin Not specified Evaluate safety and tolerability of IV vitamin C.
NCT00441207 C treatment in patients with solid Observe evidence of tumor response to vitamin C
tumors and compare the level of fatigue, pain control,
quality of life before and after vitamin C.
Hoffer et al. I–II Phases I–II clinical trial of combination Dose escalation beginning with Evaluate safety and tolerability of IV vitamin C when
NCT01050621 conventional cytotoxic chemotherapy 0.9 g/kg escalating to 1.5 g/kg administered alongside cytotoxic therapies.
Asia-Pac J Clin Oncol 2014; 10: 22–37
and IV vitamin C in patients with IV two to three times per To assess tumor response.
High-dose IV vitamin C as anticancer agent
advanced cancer or hematological week, bracketing To assess effect on quality of life.
malignancy for whom cytotoxic chemotherapy Determine the effect of chemotherapy on
chemotherapy alone is only pharmacokinetics of IV vitamin C.
marginally effective
Mikines II Evaluation of cytotoxicity and genetic 20 g IV vitamin C weekly PSA change after 12–20 weekly treatments.
NCT01080352 changes of high-dose IV vitamin C Changes in bone metastases and markers of bone
infusions in castration-resistant activity (bone-specific ALP, PINP, NTX).
metastatic human prostate cancer Pharmacokinetics in elderly cancer patients.
Monti II Pilot trial of IV vitamin C in refractory IV vitamin C to achieve plasma Evaluate safety and tolerability of IV vitamin C and
NCT00626444 non-Hodgkin lymphoma (NHL) level of 300–350 mg/dL given tumor shrinkage.
three times per week
Edman I IV vitamin C in combination with 50, 75 or 100 g IV vitamin C Evaluate safety and tolerability of IV vitamin C and
NCT00954525 standard chemotherapy for pancreatic three times per week tumor shrinkage.
cancer
Drisko II Safety of oral antioxidants and IV Oral and IV vitamin C two to Evaluate safety of adding high-dose Antioxidants to
vitamin C during gyn cancer care three times per week chemotherapy in the treatment of gynecologic
(individual doses not malignancies (uterine, cervical or epithelial
specified) ovarian).
Evaluate tumor response rates in patients with
gynecologic malignancies treated with antioxidants
to include IV and oral ascorbic acid, IV
glutathione, oral mixed carotenoids, mixed
tocopherols and vitamin A.
ALP, alkaline phosphatase; IV, intravenous; NTX, N-terminal telopeptide; PINP, procollagen type I N-terminal propeptide; PSA, prostate-specific antigen.
© 2014 Wiley Publishing Asia Pty Ltd
2930 MK Wilson et al.
Table 3 Design of potential clinical trial
Factors to be considered Recommendations
Population 1. Palliative patients with no further chemotherapeutic options
2. Alongside chemotherapy in refractory disease – ideal to use alongside one specific agent
3. One tumor subtype on observation alone, for example, good prognosis renal cell cancer
4. Across tumor subtypes based on initial data from Cameron trial
Trial arms 1. Comparison of oral high-dose (low plasma concentration) vitamin C versus IV high-dose
(high plasma concentration) vitamin C
2. Three arm trial with placebo versus oral high-dose vitamin C versus IV high-dose vitamin C
3. Placebo versus high-dose IV vitamin C
4. Similar arms as described above alongside chemotherapy or radiotherapy†
Pharmacokinetics 1. Baseline and on-treatment assessment of plasma vitamin C levels
2. If alongside chemotherapy, pharmacokinetic studies of the chemotherapeutic agent
Markers of response 1. Pre- and posttreatment biopsy if easily obtainable tissue
2. Catalase genotype evaluation as subgroup analysis to determine if there is a potential target
population with increased efficacy
3. CT/MRI to assess response – timing of imaging remains controversial
4. PET-CT scan to assess tumor metabolic activity pre- and posttreatment
End points 1. Assessment of tumor response
2. Progression-free and overall survival
3. Quality of life assessment
†
Addition of vitamin C alongside chemotherapy or radiation treatment has a number of ethical concerns because of the potential for both a beneficial
or detrimental interaction. Vitamin C could be studied in a population who have developed chemoresistance and have no further treatment options.
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
in good prognosis RCC or indolent low-grade NHL. chemotherapeutic agent. In vitro and in vivo data suggest
Regardless of the population used, patient safety remains vitamin C may overcome chemoresistance and improve
paramount. All patients should have a G6PD screen and chemosensitivity.11,70–73 This has been demonstrated in
caution should be exercised in patients with a history of vivo in pancreatic cancer cell lines in combination with
renal stones. gemcitabine, making this a potential population to start
Baseline and on-treatment vitamin C levels should with.11
be assessed and used to titrate dose to achieve a concen- Biopsies pre- and post treatment may help identify
tration of at least 10 mM based on in vitro data.8 predictors and markers of response. This has not been
This would help improve knowledge on the pharmaco- performed in the trials to date. Method and timing
kinetic properties of vitamin C and help establish a of assessment of tumor response is hindered by our
dose–response relationship. limited understanding of the mechanism of action
Based on experience gained from chemotherapeutic and lack of specific biomarkers. Improvement of our
agents, further delineation of the mechanism of action translational knowledge is critical for future research.
may identify a target population with higher response Fluorodeoxyglucose-positron emission tomography
rates. If vitamin C acts via H2O2 formation, population- could be used as a surrogate marker of response by
based differences in the genotype and phenotype of cata- assessing the effect of high-dose vitamin C on tumor
lase expression and activity may be relevant.80 Those metabolism. There is evidence to support the use of
with low levels of catalase activity may be more sensitive PET to assess response to other modalities such as
to the effect and toxicity of high-dose vitamin C. Assess- chemotherapy and chemoradiation.81
ment of this in a clinical trial would help determine if
this theoretical sensitivity translates to clinical response.
CONCLUSION
It remains uncertain whether the use of vitamin C in
conjunction with chemotherapy and radiotherapy has a Although the rates of utilization of vitamin C therapy
beneficial or detrimental interaction. Trials using vitamin remain uncertain, its popularity has increased over
C alongside chemotherapy need to include analyses of the the years since the first suggestion of its chemo-
pharmacokinetic properties of both vitamin C and the therapeutic activity in the 1970s. Although there is
© 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37High-dose IV vitamin C as anticancer agent 31
currently no definitive evidence that IV vitamin C 2 McCormick WJ. Ascorbic acid as a chemotherapeutic
improves quality of life, progression-free or overall sur- agent. Arch Pediatr 1952; 69 (4): 151–5.
vival, the published RCT data do not negate potential 3 Cameron E, Pauling L. Supplemental ascorbate in the sup-
benefit based on an improved understanding of vitamin C portive treatment of cancer: prolongation of survival times
in terminal human cancer. Proc Natl Acad Sci U S A 1976;
pharmacokinetics.
73 (10): 3685–9.
The pharmacokinetic properties of IV and oral vitamin
4 Creagan ET, Moertel CG, O’Fallon JR et al. Failure of
C are critical in interpreting the data to date. Based on in high-dose vitamin C (ascorbic acid) therapy to benefit
vitro data, it is now recognized that the plasma levels patients with advanced cancer. A controlled trial. N Engl J
necessary for cytotoxicity requires IV dosing. However, a Med 1979; 301 (13): 687–90.
phase I trial of 24 patients did not demonstrate objective 5 Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell
responses despite using IV doses of up to 1.5 g/kg.58 MJ, Ames MM. High-dose vitamin C versus placebo in the
Vitamin C is well known for its antioxidant activity, treatment of patients with advanced cancer who have had
but it is the proposed pro-oxidant activity at high con- no prior chemotherapy. A randomized double-blind com-
centrations that remains controversial. This situation parison. N Engl J Med 1985; 312 (3): 137–41.
is perpetuated by the lack of a defined mechanism of 6 Tschetter L, Creagan E, O’Fallon J et al. A community-
based study of vitamin C (ascorbic acid) in patients with
action. While intracellular and extracellular generation
advanced cancer. Proc Am Soc Clin Oncol 1983; 2: abstract
of H2O2 is the most common theory, clarification of this
92.
and determination whether this will translate to a clini- 7 Levine M, Conry-Cantilena C, Wang Y et al. Vitamin C
cal benefit is critical in future research. pharmacokinetics in healthy volunteers: evidence for
Although high-dose IV vitamin C appears relatively a recommended dietary allowance. Proc Natl Acad Sci
innocuous given alone, it does have the potential to U S A 1996; 93 (8): 3704–9.
cause harm in patients with G6PD deficiency and previ- 8 Padayatty SJ, Sun H, Wang Y et al. Vitamin C pharmaco-
ous renal stones. It remains uncertain whether vitamin C kinetics: implications for oral and intravenous use. Ann
is clinically safe when given alongside chemotherapy Intern Med 2004; 140 (7): 533–7.
and radiotherapy. 9 Chen Q, Espey MG, Sun AY et al. Pharmacologic doses of
Despite 40 years of research since the initial reports ascorbate act as a prooxidant and decrease growth of
aggressive tumor xenografts in mice. Proc Natl Acad Sci
on high-dose IV vitamin C, its use remains controversial.
U S A 2008; 105 (32): 11105–9.
The methodology exists to determine if high-dose IV
10 Takemura Y, Satoh M, Satoh K, Hamada H, Sekido Y,
vitamin C does have an anticancer effect, but the ability Kubota S. High dose of ascorbic acid induces cell death in
to design and conduct studies is impaired by the lack of mesothelioma cells. Biochem Biophys Res Commun 2010;
a consistent scientific rationale, the ready availability 394 (2): 249–53.
and the use of this agent by practitioners already con- 11 Espey MG, Chen P, Chalmers B et al. Pharmacologic ascor-
vinced by current evidence. This makes a placebo- bate synergizes with gemcitabine in preclinical models of
controlled trial difficult. pancreatic cancer. Free Radic Biol Med 2011; 50 (11):
There are highly polarized views on the use of high- 1610–9.
dose vitamin C for cancer treatment, with passionate 12 Levine M, Padayatty SJ, Espey MG. Vitamin C: a
advocates balanced by passionate critics. This is a key concentration–function approach yields pharmacology and
therapeutic discoveries. Adv Nutr 2011; 2 (2): 78–88.
reason for why carefully controlled clinical trials, rather
13 Cameron E, Pauling L. Ascorbic acid and the glycosamino-
than a review of the literature, are needed to obtain a
glycans. An orthomolecular approach to cancer and other
clear view of this field. diseases. Oncology 1973; 27 (2): 181–92.
14 Shapiro SS, Bishop M, Kuenzig W, Tkaczevski V, Kamm
JJ. Effect of ascorbic acid on hyaluronidase inhibitor.
ACKNOWLEDGMENT Nature 1975; 253 (5491): 479–80.
No funding for this project. 15 Vojdani A, Ghoneum M. In vivo effect of ascorbic acid on
enhancement of human natural killer cell activity. Nutr Res
1993; 13: 753–64.
REFERENCES 16 Herberman R. Possible role of natural killer cells in host
resistance against tumors and diseases. Clin Immunol
1 McCormick WJ. Cancer: the preconditioning factor in Allergy 1983; 3: 479–85.
pathogenesis; a new etiologic approach. Arch Pediatr 17 Heuser G, Vojdani A. Enhancement of natural killer cell
1954; 71 (10): 313–22. activity and T and B cell function by buffered vitamin C in
Asia-Pac J Clin Oncol 2014; 10: 22–37 © 2014 Wiley Publishing Asia Pty Ltd32 MK Wilson et al.
patients exposed to toxic chemicals: the role of protein increased hypoxia-inducible factor-1 activity and an
kinase-C. Immunopharmacol Immunotoxicol 1997; 19 (3): aggressive tumor phenotype in endometrial cancer. Cancer
291–312. Res 2010; 70 (14): 5749–58.
18 Siegel BV, Morton JI. Vitamin C and immunity: natural 34 Brown LM, Cowen RL, Debray C et al. Reversing hypoxic
killer (NK) cell factor. Int J Vitam Nutr Res 1983; 53 (2): cell chemoresistance in vitro using genetic and small mol-
179–83. ecule approaches targeting hypoxia inducible factor-1. Mol
19 Chen Q, Espey MG, Sun AY et al. Ascorbate in pharma- Pharmacol 2006; 69 (2): 411–8.
cologic concentrations selectively generates ascorbate 35 Song X, Liu X, Chi W et al. Hypoxia-induced resistance to
radical and hydrogen peroxide in extracellular fluid in vivo. cisplatin and doxorubicin in non-small cell lung cancer is
Proc Natl Acad Sci U S A 2007; 104 (21): 8749–54. inhibited by silencing of HIF-1alpha gene. Cancer
20 Rhee SG. Cell signaling. H2O2, a necessary evil for cell Chemother Pharmacol 2006; 58 (6): 776–84.
signaling. Science 2006; 312 (5782): 1882–3. 36 Chen C, Sun J, Liu G, Chen J. Effect of small interference
21 Chatterjee M, Saluja R, Kumar V et al. Ascorbate sustains RNA targeting HIF-1alpha mediated by rAAV combined L:
neutrophil NOS expression, catalysis, and oxidative burst. -ascorbate on pancreatic tumors in athymic mice. Pathol
Free Radic Biol Med 2008; 45 (8): 1084–93. Oncol Res 2009; 15 (1): 109–14.
22 Deubzer B, Mayer F, Kuci Z et al. H(2)O(2)-mediated 37 Cameron E, Campbell A. The orthomolecular treatment of
cytotoxicity of pharmacologic ascorbate concentrations to cancer. II. Clinical trial of high-dose ascorbic acid supple-
neuroblastoma cells: potential role of lactate and ferritin. ments in advanced human cancer. Chem Biol Interact
Cell Physiol Biochem 2010; 25 (6): 767–74. 1974; 9 (4): 285–315.
23 Chen Q, Espey MG, Krishna MC et al. Pharmacologic 38 Cameron E, Campbell A, Jack T. The orthomolecular
ascorbic acid concentrations selectively kill cancer cells: treatment of cancer. III. Reticulum cell sarcoma: double
action as a pro-drug to deliver hydrogen peroxide to complete regression induced by high-dose ascorbic acid
tissues. Proc Natl Acad Sci U S A 2005; 102 (38): 13604–9. therapy. Chem Biol Interact 1975; 11 (5): 387–93.
24 Tsukaguchi H, Tokui T, Mackenzie B et al. A family of 39 Cameron E, Pauling L. Supplemental ascorbate in the sup-
mammalian Na+-dependent L-ascorbic acid transporters. portive treatment of cancer: reevaluation of prolongation
Nature 1999; 399 (6731): 70–5. of survival times in terminal human cancer. Proc Natl Acad
25 Washko PW, Wang Y, Levine M. Ascorbic acid recycling in Sci U S A 1978; 75 (9): 4538–42.
human neutrophils. J Biol Chem 1993; 268 (21): 15531–5. 40 Wittes RE. Vitamin C and cancer. N Engl J Med 1985; 312
26 May JM, Li L, Qu ZC, Huang J. Ascorbate uptake and (3): 178–9.
antioxidant function in peritoneal macrophages. Arch 41 Cameron E. Vitamin C for cancer. N Engl J Med 1980; 302
Biochem Biophys 2005; 440 (2): 165–72. (5): 299.
27 May JM, Qu ZC. Transport and intracellular accumulation 42 Pauling L. Vitamin C therapy of advanced cancer. N Engl
of vitamin C in endothelial cells: relevance to collagen J Med 1980; 302 (12): 694–5.
synthesis. Arch Biochem Biophys 2005; 434 (1): 178–86. 43 Cameron E, Campbell A. Innovation vs. quality control:
28 Bruegge K, Jelkmann W, Metzen E. Hydroxylation of an “unpublishable” clinical trial of supplemental ascor-
hypoxia-inducible transcription factors and chemical com- bate in incurable cancer. Med Hypotheses 1991; 36 (3):
pounds targeting the HIF-alpha hydroxylases. Curr Med 185–9.
Chem 2007; 14 (17): 1853–62. 44 Murata A, Morishige F, Yamaguchi H. Prolongation of
29 Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev survival times of terminal cancer patients by administration
Cancer 2003; 3 (10): 721–32. of large doses of ascorbate. Int J Vitam Nutr Res Suppl
30 Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, 1982; 23: 103–13.
Currie MJ. Modulation of hypoxia-inducible factor-1 45 Riordan HD, Riordan NH, Jackson JA et al. Intravenous
alpha in cultured primary cells by intracellular ascorbate. vitamin C as a chemotherapy agent: a report on clinical
Free Radic Biol Med 2007; 42 (6): 765–72. cases. P R Health Sci J 2004; 23 (2): 115–8.
31 Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham 46 Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ,
CH. Hypoxia-induced resistance to anticancer drugs is Levine M. Intravenously administered vitamin C as cancer
associated with decreased senescence and requires hypoxia- therapy: three cases. CMAJ 2006; 174 (7): 937–42.
inducible factor-1 activity. Mol Cancer Ther 2008; 7 (7): 47 Riordan H, Jackson J, Schultz M. Case study: high-dose
1961–73. intravenous vitamin C in the treatment of a patient with
32 Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, adenocarcinoma of the kidney. J Orthomol Med 1990; 5
Semenza GL. Expression of hypoxia-inducible factor (1): 5–7.
1alpha in brain tumors: association with angiogenesis, 48 Jackson J, Riordan H, Hunninghake R, Riordan N. High
invasion, and progression. Cancer 2000; 88 (11): 2606–18. dose intravenous vitamin C and long time survival of a
33 Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, patient with cancer of the head of the pancreas. J Orthomol
Vissers MC. Low ascorbate levels are associated with Med 1995; 10 (2): 87–8.
© 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37High-dose IV vitamin C as anticancer agent 33 49 Drisko JA, Chapman J, Hunter VJ. The use of antioxidants 65 Campbell A, Jack T. Acute reactions to mega ascorbic acid with first-line chemotherapy in two cases of ovarian cancer. therapy in malignant disease. Scott Med J 1979; 24 (2): J Am Coll Nutr 2003; 22 (2): 118–23. 151–3. 50 Lokich J. Spontaneous regression of metastatic renal 66 Richardson MA, Sanders T, Palmer JL, Greisinger A, cancer. Case report and literature review. Am J Clin Oncol Singletary SE. Complementary/alternative medicine use in 1997; 20 (4): 416–8. a comprehensive cancer center and the implications for 51 Assouline S, Miller WH. High-dose vitamin C therapy: oncology. J Clin Oncol 2000; 18 (13): 2505–14. renewed hope or false promise? CMAJ 2006; 174 (7): 67 Ernst E, Cassileth BR. The prevalence of complementary/ 956–7. alternative medicine in cancer: a systematic review. Cancer 52 Winter WE 3rd, Maxwell GL, Tian C et al. Prognostic 1998; 83 (4): 777–82. factors for stage III epithelial ovarian cancer: a Gynecologic 68 Rausch SM, Winegardner F, Kruk KM et al. Complemen- Oncology Group Study. J Clin Oncol 2007; 25 (24): tary and alternative medicine: use and disclosure in radia- 3621–7. tion oncology community practice. Support Care Cancer 53 Heintz AP, Odicino F, Maisonneuve P et al. Carcinoma of 2011; 19 (4): 521–9. the ovary. FIGO 26th Annual Report on the Results of 69 Golde DW. Vitamin C in cancer. Integr Cancer Ther 2003; Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2 (2): 158–9. 2006; 95 (Suppl 1): S161–92. 70 Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise 54 Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach LH. Ascorbic acid enhances arsenic trioxide-induced cyto- L, Beuth J. Intravenous vitamin C administration improves toxicity in multiple myeloma cells. Blood 2001; 98 (3): quality of life in breast cancer patients during chemo-/ 805–13. radiotherapy and aftercare: results of a retrospective, mul- 71 Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs ticentre, epidemiological cohort study in Germany. In Vivo D, Bruckner HW. Ascorbic acid (vitamin C) improves the 2011; 25 (6): 983–90. antineoplastic activity of doxorubicin, cisplatin, and pacli- 55 Albers G, Echteld MA, de Vet HC, Onwuteaka-Philipsen taxel in human breast carcinoma cells in vitro. Cancer Lett BD, van der Linden MH, Deliens L. Evaluation of quality- 1996; 103 (2): 183–9. of-life measures for use in palliative care: a systematic 72 Song EJ, Yang VC, Chiang CD, Chao CC. Potentiation of review. Palliat Med 2010; 24 (1): 17–37. growth inhibition due to vincristine by ascorbic acid in a 56 Yeom CH, Jung GC, Song KJ. Changes of terminal cancer resistant human non-small cell lung cancer cell line. Eur J patients’ health-related quality of life after high dose Pharmacol 1995; 292 (2): 119–25. vitamin C administration. J Korean Med Sci 2007; 22 (1): 73 Reddy VG, Khanna N, Singh N. Vitamin C augments 7–11. chemotherapeutic response of cervical carcinoma HeLa 57 Casciari JJ, Riordan NH, Schmidt TL, Meng XL, Jackson cells by stabilizing P53. Biochem Biophys Res Commun JA, Riordan HD. Cytotoxicity of ascorbate, lipoic acid, 2001; 282 (2): 409–15. and other antioxidants in hollow fibre in vitro tumours. Br 74 Koch CJ, Biaglow JE. Toxicity, radiation sensitivity modi- J Cancer 2001; 84 (11): 1544–50. fication, and metabolic effects of dehydroascorbate and 58 Hoffer LJ, Levine M, Assouline S et al. Phase I clinical trial ascorbate in mammalian cells. J Cell Physiol 1978; 94 (3): of i.v. ascorbic acid in advanced malignancy. Ann Oncol 299–306. 2008; 19 (11): 1969–74. 75 Prasad KN, Sinha PK, Ramanujam M, Sakamoto A. 59 Riordan HD, Casciari JJ, Gonzalez MJ et al. A pilot clinical Sodium ascorbate potentiates the growth inhibitory effect study of continuous intravenous ascorbate in terminal of certain agents on neuroblastoma cells in culture. Proc cancer patients. P R Health Sci J 2005; 24 (4): 269–76. Natl Acad Sci U S A 1979; 76 (2): 829–32. 60 Rivers JM. Safety of high-level vitamin C ingestion. Ann N 76 Witenberg B, Kletter Y, Kalir HH et al. Ascorbic acid Y Acad Sci 1987; 498: 445–54. inhibits apoptosis induced by X irradiation in HL60 61 Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. myeloid leukemia cells. Radiat Res 1999; 152 (5): 468–78. Criteria and recommendations for vitamin C intake. JAMA 77 Williams KJ, Telfer BA, Xenaki D et al. Enhanced response 1999; 281 (15): 1415–23. to radiotherapy in tumours deficient in the function of 62 Campbell GD Jr, Steinberg MH, Bower JD. Letter: ascorbic hypoxia-inducible factor-1. Radiother Oncol 2005; 75 (1): acid-induced hemolysis in G-6-PD deficiency. Ann Intern 89–98. Med 1975; 82 (6): 810. 78 Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation acti- 63 Rees DC, Kelsey H, Richards JD. Acute haemolysis vates HIF-1 to regulate vascular radiosensitivity in tumors: induced by high dose ascorbic acid in glucose-6-phosphate role of reoxygenation, free radicals, and stress granules. dehydrogenase deficiency. BMJ 1993; 306 (6881): 841–2. Cancer Cell 2004; 5 (5): 429–41. 64 McAllister CJ, Scowden EB, Dewberry FL, Richman A. 79 Shimpo K, Nagatsu T, Yamada K et al. Ascorbic acid and Renal failure secondary to massive infusion of vitamin C. adriamycin toxicity. Am J Clin Nutr 1991; 54 (6 Suppl): JAMA 1984; 252 (13): 1684. 1298S–301S. Asia-Pac J Clin Oncol 2014; 10: 22–37 © 2014 Wiley Publishing Asia Pty Ltd
34 MK Wilson et al. 80 Ahn J, Nowell S, McCann SE et al. Associations between 90 Du J, Martin SM, Levine M et al. Mechanisms of catalase phenotype and genotype: modification by epide- ascorbate-induced cytotoxicity in pancreatic cancer. Clin miologic factors. Cancer Epidemiol Biomarkers Prev 2006; Cancer Res 2010; 16 (2): 509–20. 15 (6): 1217–22. 91 Sestili P, Brandi G, Brambilla L, Cattabeni F, Cantoni O. 81 Quarles van Ufford HM, van Tinteren H, Stroobants SG, Hydrogen peroxide mediates the killing of U937 tumor Riphagen II, Hoekstra OS. Added value of baseline 18F- cells elicited by pharmacologically attainable concentra- FDG uptake in serial 18F-FDG PET for evaluation of tions of ascorbic acid: cell death prevention by extracellular response of solid extracerebral tumors to systemic cyto- catalase or catalase from cocultured erythrocytes or fibro- toxic neoadjuvant treatment: a meta-analysis. J Nucl Med blasts. J Pharmacol Exp Ther 1996; 277 (3): 1719–25. 2010; 51 (10): 1507–16. 92 Heaney ML, Gardner JR, Karasavvas N et al. Vitamin C 82 Poydock ME. Effect of combined ascorbic acid and B-12 antagonizes the cytotoxic effects of antineoplastic drugs. on survival of mice with implanted Ehrlich carcinoma and Cancer Res 2008; 68 (19): 8031–8. L1210 leukemia. Am J Clin Nutr 1991; 54 (6 Suppl): 93 Noto V, Taper HS, Jiang YH, Janssens J, Bonte J, De Loecker 1261S–5S. W. Effects of sodium ascorbate (vitamin C) and 2-methyl- 83 Verrax J, Calderon PB. Pharmacologic concentrations of 1,4-naphthoquinone (vitamin K3) treatment on human ascorbate are achieved by parenteral administration and tumor cell growth in vitro. I. Synergism of combined vitamin exhibit antitumoral effects. Free Radic Biol Med 2009; 47 C and K3 action. Cancer 1989; 63 (5): 901–6. (1): 32–40. 94 Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can 84 Pollard HB, Levine MA, Eidelman O, Pollard M. Pharma- be sensitized to undergo growth inhibition and apoptosis cological ascorbic acid suppresses syngeneic tumor growth by arsenic trioxide through modulation of the glutathione and metastases in hormone-refractory prostate cancer. In redox system. Blood 1999; 93 (1): 268–77. Vivo 2010; 24 (3): 249–55. 95 Prasad KN, Sinha PK, Ramanujam M, Sakamoto A. 85 Park CH, Kimler BF. Growth modulation of human leuke- Sodium ascorbate potentiates the growth inhibitory effect mic, preleukemic, and myeloma progenitor cells by of certain agents on neuroblastoma cells in culture. Proc L-ascorbic acid. Am J Clin Nutr 1991; 54 (6 Suppl): Natl Acad Sci U S A 1979; 76 (2): 829–32. 1241S–6S. 96 Abdel-Latif MM, O’Riordan JM, Ravi N, Kelleher D, 86 Park CH, Amare M, Savin MA, Hoogstraten B. Growth Reynolds JV. Activated nuclear factor-kappa B and cyto- suppression of human leukemic cells in vitro by L-ascorbic kine profiles in the esophagus parallel tumor regression acid. Cancer Res 1980; 40 (4): 1062–5. following neoadjuvant chemoradiotherapy. Dis Esophagus 87 Leung PY, Miyashita K, Young M, Tsao CS. Cytotoxic 2005; 18 (4): 246–52. effect of ascorbate and its derivatives on cultured malig- 97 Taper HS, Roberfroid M. Non-toxic sensitization of cancer nant and nonmalignant cell lines. Anticancer Res 1993; 13 chemotherapy by combined vitamin C and K3 pretreat- (2): 475–80. ment in a mouse tumor resistant to oncovin. Anticancer 88 Bram S, Froussard P, Guichard M et al. Vitamin C prefer- Res 1992; 12 (5): 1651–4. ential toxicity for malignant melanoma cells. Nature 1980; 98 Kassouf W, Highshaw R, Nelkin GM, Dinney CP, Kamat 284 (5757): 629–31. AM. Vitamins C and K3 sensitize human urothelial tumors 89 De Laurenzi V, Melino G, Savini I, Annicchiarico- to gemcitabine. J Urol 2006; 176 (4 Pt 1): 1642–7. Petruzzelli M, Finazzi-Agro A, Avigliano L. Cell death by 99 Qazilbash MH, Saliba RM, Nieto Y et al. Arsenic trioxide oxidative stress and ascorbic acid regeneration in human with ascorbic acid and high-dose melphalan: results of a neuroectodermal cell lines. Eur J Cancer 1995; 31A (4): phase II randomized trial. Biol Blood Marrow Transplant 463–6. 2008; 14 (12): 1401–7. © 2014 Wiley Publishing Asia Pty Ltd Asia-Pac J Clin Oncol 2014; 10: 22–37
You can also read