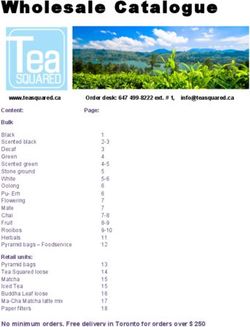
Bloodborne Pathogens (BBP) Training for the Researcher - 2008 Update for employees with
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Bloodborne Pathogens (BBP) Training
for the Researcher
Update for employees with
potential exposure to blood or
other potentially infectious
materials (OPIM) - Safety
precautions to prevent infections
2008Louisiana State University A&M
Louisiana AgCenter
Office of Occupational & Environmental Safety (OES)
Public Safety Building, Suite 126
South Stadium Drive
Phone: (225) 578-5640 Fax: (225) 578-7489
Biological Training
Safety Manager Manager
Gregory V.
Pat F. West
Hayes, DrPH
(225) 578-4658 (225) 578-0534
ghayes@lsu.edu
pwest@lsu.edu
2Bloodborne Pathogens (BBP)
The following training meets requirements
set forth by the OSHA Bloodborne Pathogens
Standard. This module and accompanied quiz is a
self-study designed to provide a review of the
following:
Understanding bloodborne pathogens (BBP) and the modes of
transmission
Understanding the epidemiology of bloodborne diseases
Recognizing workers and tasks that may involve exposure
Learning containment practices for safe handling of BBP
Awareness of the need for Exposure Control Plans
Acquiring Information on the Hepatitis B vaccineBloodborne Pathogens Standard
This training was based upon Federal regulations and
guidelines:
OSHA (1991)- “Occupational Exposure to
Bloodborne Pathogens” (29 CFR 1910.1030)
NIOSH/CDC - Preventing Needle stick
Injures in Health-Care Setting
CDC - Exposure to Blood: What Health-Care
Workers Need to Know
5What are Bloodborne Pathogens (BBP)?
Any pathogenic microorganisms or OPIM (other
potentially infectious materials) present in human
blood that can cause disease in humans.
Primary focus in our setting is HBV, HCV, and
HIV which will be detailed further in this
presentation.
Other examples of BBP include microorganisms
that cause: malaria, syphilis, babesiosis, brucellosis,
tularemia, leptospirosis, arbovirus infections ( i.e., Dengue),
relapsing fever, Creutzfeldt-Jakob disease, viral hemorrhagic
fever and many many more.
6Working with human or primate cell lines?
LSU developed a policy on the use of human &
primate cell lines in 2006
All cell and organ cultures of
human origin, including well
established cell lines as well as
primate cell lines, shall be
handled in accordance with the
OSHA Bloodborne Pathogens
Standard and under Biosafety
Level 2 (BSL2) containment. All
University personnel working
with these cultures shall maintain
a written record of their annual
training as required under the
OSHA Bloodborne Pathogens
Standard. http://appl003.lsu.edu/PubSafety/oes.nsf/$Content/Bloodborne+Pathogens+and+Uni
versal+Precautions+at+LSU?OpenDocument
7Exposure to Blood
For some employees the potential for
infection from occupational exposure
to blood or other potentially infectious
materials (OPIM) is high, especially
from:
o Needle sticks
o Cuts from other
sharp instruments
contaminated with blood
o And through eye, nose,
mouth or skin contacts
with patients
8OPIM includes the following:
9 Synovial, pleural, 9 Any unfixed tissue or organ
pericardial, and from a human
peritoneal fluid 9 Any body fluid visibly
contaminated with blood
9 Cerebrospinal fluid
9 All body fluid where it is
9 Semen
difficult to distinguish
9 Vaginal secretions between body fluids
9Cell or tissue cultures
9 Amniotic fluid
9 Saliva (in dental
procedures)
9Risk Factors for Infection
Most exposure does not result in
infection. The risk of infection
may vary with certain factors:
Pathogenicity of organism
Dose (how much blood or
infectious agent)
Route of entry (injection vs.
contact with mucous
membrane or open wound)
Host susceptibility
Work practices
10Duties that might put you at risk for an
occupational exposure:
o Disposing of waste contaminated with blood
or OPIM
o Transporting blood or OPIM
o Working in a laboratory where equipment or work
benches can become contaminated
o Handling containers of infectious wastes
o Cleaning blood spills, including dried blood
o Handling laundry that contains sharps or is soiled
with blood or OPIM
o Performing lifesaving procedures
o Working in a faulty biological safety cabinet
11Duties that might put you at risk for an
occupational exposure - continued:
o Performing a blood draw from human patients or
animals
o Processing blood for experimentation
o Using human or animal blood or body fluids in
experimentation
o Using unfixed tissue in preparations or
experimentation
o Cleaning glassware contaminated with blood or
OPIM
o Performing flow cytometry with unfixed cells
12Modes of transmission of BBP
Percutaneous - the direct inoculation of
infectious material by piercing through the skin
barrier (needle stick or other accidental injury
with a sharp, contaminated object)
Penetration by contaminated sharps is the
most common mode of transmission of
bloodborne pathogens in the workplace.
13Modes of transmission of BBP
Direct inoculation - exposure of blood or
OPIM to pre-existing lesions, cuts,
abrasions, or rashes (dermatitis) provides
a route of entry into the body.
Mucous membrane contact - splashing
blood or serum into an individual's
unprotected eyes, nose, or mouth in
clinical or laboratory settings poses a
genuine risk of infection.
14Human Immunodeficiency virus (HIV)
HIV is the virus that causes AIDS
(Acquired Immune Deficiency Syndrome).
Once a person has been infected with computer generated art quality
graphics of HIV was done by
HIV, it may be many years before AIDS Russell Kightley of Canberra,
Australia.
actually develops.
HIV kills or damages cells in the body’s
immune system, gradually destroying the
body’s ability to fight infection and certain
cancers.
15HIV Infection
HIV viruses establish a chronic infection of
human CD4+ cells: “helper” T-lymphocytes
and macrophage
Currently there is no vaccine available and
drug therapies are effective at limiting
progression of disease but not curing
infection
16HIV Transmission
Exposure to infected blood or blood
products:
¾ transfusions, mainly in the developing world today
¾ intravenous drug use, sharing of needles (main
transmission in eastern Europe and former Soviet
Union states)
¾ accidental needle-sticks or exposure of blood to
open cuts or scrapes
17Early Signs / Symptoms of HIV infection
Initial signs are mononucleosis-like:
¾ swollen, tender lymph nodes
¾ fever
¾ sore throat, headache
¾ muscle aches
¾ rash, diarrhea may be present
A vigorous immune response occurs:
¾ virus levels in blood decline
Sharp decline in circulating CD4+ T-cells,
then numbers recover
18Hepatitis B Virus (HBV)
Hepatitis B is caused by a virus that attacks
the liver and can cause lifelong infection,
cirrhosis, liver cancer, liver failure, or death.
In 2003, an estimated 73,000 people were
infected with HBV. People of all ages get
hepatitis B and about 5,000 die per year of
sickness caused by HBV.
19Hepatitis B Virus
HBV infection is a well recognized
occupational risk for healthcare
personnel.
The average volume of blood inoculated
during a needle stick injury with a 22-
gauge needle is approximately 1 µl, a
quantity sufficient to contain up to 100
infectious doses of HBV.
HBV can survive outside the body at
least 7 days and still be capable of
causing infection.
20Hepatitis B Virus
About 30% of infected persons have no sign or
symptoms of HBV.
If symptoms occur, they usually begin to
appear on the average of 12 weeks (range 9-
21 weeks) after exposure to hepatitis B virus.
If you have symptoms, they might include:
• jaundice • abdominal discomfort
• dark urine • clay-colored bowel
• joint pain movements
• fatigue
• loss of appetite • nausea
21What treatment is available for HBV?
In the occupational setting, multiple doses of
Hepatitis B Immune Globulin initiated within 1
week following percutaneous exposure to
hepatitis B surface antigen-positive blood
provides an estimated 75% protection from
HBV infection.
There is no cure available for acute HBV
infection. There are antiviral drugs available
for the treatment of chronic HBV infection.
22HBV Infection
HBV is a small DNA virus in the family
Hepadnaviridae that causes both self-limiting
and chronic infections of humans:
¾ self limiting - resolve within 6 months
o most are sub-clinical
o some result in acute hepatitis
¾ persistent - a fraction of infections become
persistent and may continue for many years
or life.
o can lead to liver damage or hepatocellular
carcinoma
23Transmission of HBV
Humans are the only reservoir, and
chronic carriers are the main source
of new infections.
HBV is present in and can be
transmitted from contact with:
o blood and serum
o saliva
o semen
24Transmission of HBV
Established routes of infection:
o percutaneous transfer of blood
o mucous membrane contact with blood
o homosexual and heterosexual intercourse
o contact between mucous membranes or cuts and
environmental surfaces contaminated with virus
o neonatal transmission is mainly at birth; 5-10% of
neonatal infections may be in utero
25HBV Epidemiology
Currently there are about 8800 new cases of HBV reported each
year; estimated 80,000 total. Of these, about 10% will become
chronic carriers.
About 1.25 million people in U.S. have chronic HBV infection.
26HBV IS PREVENTABLE!
A safe & effective vaccine is available.
Hepatitis B vaccine prevents hepatitis B infection
and its serious consequences. The vaccine for HBV
is purified recombinant HbsAg
If the vaccine is administered before infection, it
prevents the development of the disease and the
carrier state in almost all individuals
Hepatitis B vaccine consists of a series of three
injections – initial, one a month later, and one six
months from the first.
Available FREE of charge from employer for at
risk employees
Principal Investigators should make the vaccine available to at
risk employees
27Hepatitis B Vaccine
FREE to employee - paid for by your
department if you are at high-risk for exposure
LSU Student Health Center administers the
vaccine and maintains the records
People who have previously been vaccinated,
have antibody to HbsAg, or are contraindicated
for medical reasons do not need the vaccine
Those who refuse the vaccine must sign a
declination form
o may reconsider vaccine at any time
28Hepatitis C Virus (HCV)
Of every 100 persons infected with HCV
about:
• 55-85 of persons might develop long-term infection
• 70 persons might develop chronic liver disease
• 5-20 persons might develop cirrhosis over a period of
20 to 30 years
• 1-5 of persons might die from the consequences of long
term infection (liver cancer or cirrhosis)
Hepatitis C is one of the leading indicators
for liver transplantation.
29Hepatitis C Virus (HCV)
80% of persons infected have no signs or
symptoms for HCV. When present,
symptoms may include:
• jaundice abdominal pain
• fatigue loss of appetite
• dark urine nausea
30Hepatitis C Virus (HCV)
Currently, there is no cure for hepatitis C,
and no effective vaccine is currently
available.
National recommendations for the control of
occupational exposure to HCV rely more on
the prevention of transmission. In addition,
several blood tests that measure either
antibodies to HCV or HCV-RNA are
available for hepatitis C screening. These
tests are useful in determining current
immune status and monitoring ongoing
infection.
3132
Epidemiology
Types of employees who have the potential for infection
from occupational exposure to blood or OPIM are:
o Healthcare workers
o Custodial and
maintenance workers
who respond to spills
o Public Safety workers
o Laboratory workers who
work with infectious
agents
33Epidemiology
Bloodborne Pathogens can
enter your body through:
o Open cuts, nicks, and skin abrasions.
o Mucous membranes of your mouth,
eyes, and nose.
o Indirect transmission - touching a
contaminated surface and then
touching your mouth, eyes or
open skin.
o Accidental injury by a contaminated
sharp object.
34Compliance Control
Occupational Exposure Prevention
The risk of occupational exposure can be
minimized or eliminated using a
combination of standard precautions, and
personal hygiene, personal protective
equipment, work practice controls,
engineering controls, training, medical
surveillance, vaccination, warning signs or
labels, and other provisions described in
this section.
35Standard Precautions
Universal
Precautions
• All human/primate blood, untreated
tissues, cell lines and bodily fluids are
treated as infectious
o Treat all blood and bodily as if they are contaminated.
o All body fluids must be considered as potentially infectious
materials.
o Proper cleanup and decontamination
o Always wear appropriate PPE
o Replace PPE that is torn or punctured
o Remove PPE before leaving the work area
36Employee Responsibilities
Completing training as required
Following an Exposure Control Plan
developed by your program or Principal
Investigator
Using work practices, engineering controls,
and personal protective equipment as outlined
in the Exposure Control Plan
Obtaining the HBV vaccine if advised by your
supervisor
37Employee Responsibilities Continued
Reporting exposure incidents to your supervisor
Pursuing follow-up care after an occupational
exposure
38Compliance Control Methods
Personal Hygiene
o Do not touch anything that is contaminated,
such as sharps or body fluids.
o Take care to minimize splashing of all
infectious materials.
o Eating, drinking, smoking, applying
cosmetics or lip balm, and handling contact
lenses are prohibited in areas where there is
a potential for occupational exposure.
39Compliance Control Methods
Personal Hygiene Cont.
Use CDC guidelines for
hand hygiene:
o If hands are not
visibly soiled, use
alcohol gel
o When hands are
visibly soiled, wash
hands with soap
and water
o Always wash your
hands before eating
and after using the
restroom
40Hand washing
Employees must wash their hands
immediately or as soon as feasible after
removal of gloves or other personal
protective equipment.
Wash as soon as possible if gross
contamination occurs
Alternate methods:
Antiseptic towelettes
Waterless hand washing gels
41Compliance Control Methods
Personal Protective Equipment (PPE)
o PPE must be used to prevent potentially
infectious materials from coming in contact with
work clothes, street clothes, undergarments, skin
or mucous membranes
o Employees must wear gloves when there is
potential contact with blood, potentially
infectious materials, mucous membranes or
broken skin
o Remove gloves promptly after use, and before
touching non-contaminated items and
environmental surfaces.
42Personal Protective Equipment
Protective outer clothing
o lab coats, gowns, or aprons are required
at all times in a BBP lab
o solid-front, fluid-resistant gowns should be used for
any procedure where splashes are possible
as necessary, add hoods, caps, face protection
and disposable shoe coverings
o all protective clothing must be removed before
leaving the lab and either disposable, laundered
on-site, or autoclaved before removal from site
43Personal Protective Equipment (PPE)
PPE is appropriate only if it does not permit
blood/OPIM to pass through and/or reach the
employee’s clothing, skin, eyes, mouth, or other
mucous membranes under normal use.
44Personal Protective Equipment
Gloves (latex or non-latex)
o When to use them:
when there is reasonable anticipation
of employee hand contact with blood, OPIM,
mucous membranes, or non-intact skin
when performing vascular access procedures
when handling or touching contaminated
surfaces or items.
o Remove prior to leaving the work area and
discard as biohazard waste
45Specific Practices & Techniques
Personal Protective Equipment for employees (PPE)
When there is occupational exposure, the employer shall provide
at no cost to the employee, appropriate PPE
Gloves, face shields or masks, eye protection, gowns, aprons,
laboratory coats, etc.
The employer is responsible for cleaning, laundering, disposal
and replacement of PPE at no cost to employee
Laundry:
o Contaminated laundry shall be bagged
o It shall not be sorted or rinsed at the location of use
o Contaminated laundry shall be transported in bags labeled
with proper warnings and symbols
o Wear proper PPE when handling contaminated laundry
46Latex Allergies
Latex gloves have proven effective in
preventing transmission of many infectious
diseases to health care workers.
However, for some workers, exposures to
latex may result in allergic reactions.
For further reading:
http://www.cdc.gov/niosh/topics/latex/
4748
Work Practice Controls
Mouth pipetting is not
permitted.
Work surfaces should be kept
free of potential hazards.
49Sharps Management
The use of sharps in bloodborne pathogen labs is
responsible for >90% of researcher exposures; mainly
needle-sticks.
50Sharps Management
Keep sharps container upright
readily available in the work area
Never place sharps into the regular trash
Use a leak-proof, puncture-resistant
sharps container labeled with the biohazard
symbol
Do not overfill - dispose of sharps container as
biohazard waste when it is 2/3 full
51Waste Management
Liquid wastes with low numbers of
pathogens may be decontaminated
by exposure to chemical
disinfectant, and discarded by
sanitary sewer.
Culture fluids and other materials expected to have
large numbers of pathogens should be autoclaved
before discard.
52Waste Management
Solid wastes should be collected into
two layers of autoclavable biohazard
bags, placed within leak-proof,
labeled secondary containers
Collection bags should be removed
from secondary containers before
overflowing and only at the time of
decontamination
All laboratory wastes should be
autoclaved before disposal into the
waste stream
53Disinfection
Cleaning and disinfection of
work surfaces should be done
after completion of each procedure
and at the end of each work day.
A variety of chemical agents are
effective against most bloodborne
pathogens: iodophors, phenolics,
alcohol, diluted bleach (10% v/v).
The presence of blood or other
organic material can limit the
effectiveness of most chemical
agents.
54Spill Specific Practices &
Techniques
Surface Contamination - Spill Control:
Define and isolate spill area
Put on proper personnel protective equipment - Includes gloves,
gowns, aprons, laboratory coats, face shields or masks, eye
protection, etc.
Remove glass/sharps with forceps or scoop
Place paper towel on spill surface and wet the paper towel with at
least 10% bleach solution
Allow for ADEQUATE CONTACT TIME - at least 15 minutes
Remove towel and wipe clean - Repeat applying disinfectant to
towel surface and allow contact time - Clean area with soapy
water
Properly dispose of spilled materials into biomedical waste
container
55Spill Specific Practices & Techniques
Recommended Spill Kit Contents
Fresh sodium hypochlorite solution (bleach) - use for
general spill (at least 10% - 1:9)
Personnel protective equipment
o Gloves, gowns, laboratory coats, face shields or
masks, eye protection, etc.
o Brushes, dust pan, tongs or forceps for picking up
contaminated sharps
o Paper towels and biohazard bags
56Spill Specific Practices & Techniques
Bleach - Hypochlorite solution
Large spill:
o Use undiluted from bottle
Small spill/virus inactivation:
o Use at least 10% bleach solution
o EPA registered tuberculocidal solution
General surface disinfections:
o Use at least 5% bleach solution
Always have fresh
solution on hand
57Work Practice Controls
Absorbent lab matting
reduces the risk of
splashes if infectious
materials are spilled on
work surfaces.
Lab matting also helps
contain spills.
58Work Practice Controls
Vacuum tubes of
blood and other
potentially infectious
materials should be
covered with
absorbent matting
during opening.
59Restricted Access
Lab doors are closed when
work is in progress
PI establishes specific entry
requirements and policies
All persons enter the lab must
be made aware of the hazards
present in the lab
A biohazard warning sign is
posted at the entrance to the
lab, other signs as appropriate.
60Safety Engineered Materials
Glassware should be
avoided if possible and
replaced with plastic
tubes, flasks, etc.
Capillary tubes, if used
for micro-hematocrit
measurements, should
be made of unbreakable
plastic or glass coated
with plastic.
61Safety Engineered Materials
If needles are used, safety needles should be
substituted for standard if possible
• If other sharps are necessary, safety-
engineered substitutes should be employed
62More Examples of Engineered Sharps Safety
Devices
In
use
Retractable needle After use
technology
Retractable
lancets
Add-ons (needle covers)
Self-blunting needles
63Biological Safety Cabinets
A properly maintained and annually
certified BSC must be used for all* open
work with infectious materials in a BBP
BSL-2 research laboratory
Use of a BSC should not
substitute for protective
clothing or eye protection.
* some procedures may not be
feasible inside a cabinet; in such
cases, extra PPE may substitute.
64Training
Training should be provided by the
supervisor or Principal Investigator:
o at the time of initial employment (or transfer) for job
tasks where occupational exposure may occur
o within one year of the employee's previous training
and annually thereafter (if the employee remains in
an at-risk position)
o when changes such as modification of tasks or
institution of new tasks affect the employee's
potential for occupational exposures, and as new
standards for safe work practices evolve
65Warning Signs and Labels
Fluorescent orange or orange-red label
with word “Biohazard” and biohazard symbol in
contrasting color must be provided on:
o Containers of regulated waste
o Refrigerators/freezers used to store blood/OPIM
o Containers used to store, transport, or ship blood/OPIM
o Contaminated equipment
Red bags may be substituted for biohazard labels on
biohazardous waste bags.
66Exposure Control Plan (ECP)
LSU exposure control plan can be found at:
http://appl003.lsu.edu/PubSafety/oes.nsf/$Content/Bloodborne
+Pathogens+and+Universal?OpenDocument
There needs to be a plan developed specific to the work area.
Each employer having an employee with potential occupational
exposure shall establish a written ECP designed to eliminate or
minimize employee exposure
The plan should discuss, universal precautions,
engineering controls, work practice controls and
medical management
The plan should be reviewed annually and requires record
keeping of the standard
67Exposure Control Plan
Updates should include:
Changes in technology that reduce/eliminate exposure
(engineering controls)
Annual documentation of consideration and implementation
of safer medical devices
Input from non-managerial employees in selecting and
evaluating safer medical devices
68Specific Practices & Techniques
Personal Contamination
Alert co-workers
Flush and clean exposed skin surface with soap and water
For eye exposure - use eye-washer or flush eye with plenty
of water
For mouth exposure - use saline solution or rinse with plenty
of water
Notify supervisor for counseling and to determine proper
reporting requirements
If necessary, confidential medical evaluation and follow-up
will be made available
69Post-Exposure
Exposure Definition: “Any eye, mouth,
other mucous membrane, non-intact skin,
or parenteral contact with blood or other
potentially infectious materials resulting
from the performance of an employee’s
duties”
All work-related exposures require action!
70Post Exposure
Contact LSU Student Health Center for Post Exposure
Evaluation or for Immunizations
Student Health Center
Infirmary Road & West Chimes
Baton Rouge, LA 70803-2401
Phone: 225/578-6271
Fax: 225/578-5655
E-mail: studenthealth@lsu.edu
Immunizations:immunization@lsu.edu
71Post Exposure
Complete LSU Occupational Accident Report
Contact LSU Office of Risk Management
Office of Risk Management
Public Safety Building, Suite 124
South Stadium Road
Baton Rouge, LA 70803 -7907
Email: riskmgt@lsu.edu
Phone: 225/578-3297
Forms available at:
http://appl003.lsu.edu/pubsafety/riskmgt.nsf
/index
72Thank you for
completing the self-
study review session.
Please click below to
complete the associated quiz
Take QuizYou can also read