Activation of hypoxia-sensing pathways promotes renal ischemic preconditioning following myocardial infarction
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Am J Physiol Renal Physiol 320: F569–F577, 2021.
First published February 1, 2021; doi:10.1152/ajprenal.00476.2020
RESEARCH ARTICLE
Activation of hypoxia-sensing pathways promotes renal ischemic
preconditioning following myocardial infarction
Andrew S. Terker,1,2 Kensuke Sasaki,1,2 Juan Pablo Arroyo,1,2 Aolei Niu,1,2 Suwan Wang,1,2 Xiaofeng Fan,1,2
Yahua Zhang,1,2 Sochinweichi Nwosisi,1,2 Ming-Zhi Zhang,1,2 and Raymond C. Harris1,2,3
1
Division of Nephrology, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee; 2Vanderbilt
Center for Kidney Disease, Nashville, Tennessee; and 3Department of Veterans Affairs, Tennessee Valley Healthcare System,
Nashville, Tennessee
Abstract
Ischemic heart disease is the leading cause of death worldwide and is frequently comorbid with chronic kidney disease.
Physiological communication is known to occur between the heart and the kidney. Although primary dysfunction in either organ
can induce dysfunction in the other, a clinical entity known as cardiorenal syndrome, mechanistic details are lacking. Here, we
used a model of experimental myocardial infarction (MI) to test effects of chronic cardiac ischemia on acute and chronic kidney
injury. Surprisingly, chronic cardiac damage protected animals from subsequent acute ischemic renal injury, an effect that was
accompanied by evidence of chronic kidney hypoxia. The protection observed post-MI was similar to protection observed in a
separate group of healthy animals housed in ambient hypoxic conditions prior to kidney injury, suggesting a common mecha-
nism. There was evidence that chronic cardiac injury activates renal hypoxia-sensing pathways. Increased renal abundance of
several glycolytic enzymes following MI suggested that a shift toward glycolysis may confer renal ischemic preconditioning. In
contrast, effects on chronic renal injury followed a different pattern, with post-MI animals displaying worsened chronic renal
injury and fibrosis. These data show that although chronic cardiac injury following MI protected against acute kidney injury via
activation of hypoxia-sensing pathways, it worsened chronic kidney injury. The results further our understanding of cardiorenal
signaling mechanisms and have implications for the treatment of heart failure patients with associated renal disease.
NEW & NOTEWORTHY Experimental myocardial infarction (MI) protects from subsequent ischemic acute kidney injury but wor-
sens chronic kidney injury. Observed protection from ischemic acute kidney injury after MI was accompanied by chronic kidney
hypoxia and increased renal abundance of hypoxia-inducible transcripts. These data support the idea that MI confers protection
from renal ischemic injury via chronic renal hypoxia and activation of downstream hypoxia-inducible signaling pathways.
acute kidney injury; cardiorenal syndrome; chronic kideny disease; hypoxia-inducible factor; myocardial infarction
INTRODUCTION Physiological explanations for cardiorenal syndrome often
cite hypoperfusion from reduced cardiac output and/or ve-
Ischemic heart disease is the leading cause of death world- nous congestion from fluid overload as overarching mecha-
wide (1). Patients with chronic cardiac dysfunction often have nisms driving renal dysfunction (7). Although these are
multiple comorbidities, and kidney disease is among the most certainly contributing factors, they may more accurately
common and most serious (2). The clinical entity wherein car- reflect the phenotype observed in the setting of acute
diac dysfunction induces renal dysfunction is known as cardi- decompensated heart failure leading to acute kidney injury
orenal syndrome (3). With the rising prevalence of both (AKI) rather than the subtler pathophysiology seen chroni-
cardiac and renal disease, clinicians encounter the cardiore- cally in patients with heart failure. Indeed, evidence from
nal syndrome more frequently in both inpatient and outpa- animal models clearly supports a role for acute cardiac dys-
tient settings. Patients with this dual-organ dysfunction function affecting renal function. It was recently reported
consume substantial financial and medical resources and that a mouse model of cardiac arrest causes acute renal fail-
have a high mortality risk (4–6). Despite the large burden car- ure and ultimately leads to chronic kidney injury weeks later
diorenal syndrome places on medical systems, a clear under- (8); however, factors driving renal injury in the setting of
standing of the physiological communication between the chronic cardiac dysfunction are likely different and remain
heart and the kidney eludes us. Limited understanding of the poorly defined. Furthermore, a clear mechanistic model of
basic physiology hinders our ability to treat these patients renal dysfunction at the cellular and molecular levels of car-
when this interorgan communication fails in disease states. diorenal syndrome continues to elude us.
Correspondence: A. S. Terker (andrew.s.terker@vumc.org); R. C. Harris (ray.harris@vumc.org).
Submitted 2 September 2020 / Revised 26 January 2021 / Accepted 26 January 2021
http://www.ajprenal.org 1931-857X/21 Published by the American Physiological Society F569
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
To gain a better understanding of cardiorenal signaling, 10 days. Animals underwent IR injury as aforementioned im-
we used a model of myocardial infarction to investigate mediately after exposure.
effects of chronic heart damage on both acute and chronic
renal injury. Unexpectedly, we found that chronic cardiac Echocardiography
dysfunction conferred protection from subsequent ischemic Mouse cardiac function was evaluated with transthoracic
AKI. Protection was accompanied by evidence of preisch- echocardiography in the conscious state using a Vevo2100
emic renal hypoxia and increased abundance of several im- Imaging System (VisualSonics). Prior to the acquisition of
portant glycolytic enzymes, suggesting that a shift to echo images, chest hair was removed by applying hair re-
anaerobic glycolysis may underlie ischemic preconditioning. moval cream. For echocardiographic examination, the
Despite amelioration of AKI, transition to chronic renal mice were gently held by their nape in the palm of one
injury and fibrosis was worse in the setting of cardiac injury, hand with the tail held between the last two fingers.
demonstrating that even modest cardiac injury drives renal Prewarmed echo transmission gel was applied to the hair-
dysfunction in the chronic setting. less chest. Parasternal long- and short-axis view at the pap-
illary muscle level and two-dimensional guided M-mode
images were recorded. Qualitative and quantitative meas-
METHODS
urements were made offline using analytical software
Animals (VisualSonics). Ejection fraction and fractional shortening
were measured in three consecutive beats according to the
All animals used were male wild-type 8- to 10-wk-old guidelines and standards of American Society of Echocar-
C57Bl/6 mice purchased from The Jackson Laboratory (Bar diography leading edge method (10).
Harbor, ME).
Transdermal Glomerular Filtration Rate Measurement
Ethical Approval
Transdermal measurement of FITC-sinistrin clearance was
All animal experiments were performed in accordance with performed to determine glomerular filtration rate (GFR) in
the guidelines and with the approval of the Institutional conscious mice, as previously described (11). The FITC-sinis-
Animal Care and Use Committee of Vanderbilt University trin half-life was calculated using a three-compartment model
Medical Center. with linear fit using MPD Studio software (MediBeacon,
Mannheim, Germany). The FITC-sinistrin half-life (in min)
Experimental Myocardial Infarction
was converted to GFR (in μL/min), as previously described
Experimental mouse MI was induced by permanent ligation (12), with correction for individual mouse body weight.
of the left anterior descending (LAD) coronary artery. Briefly,
after being anesthetized with ketamine and xylazine (80/ Blood Urea Nitrogen Measurement
10 mg/kg ip), intubated, and ventilated with a positive-pressure Blood was collected from the tail vein in heparinized tubes
ventilator (Harvard Apparatus, Holliston, MA), the heart was on the indicated days post-kidney injury. Blood was centri-
exposed via a left thoracotomy between the third and fourth fuged for 5 min at 10,000 g, and the plasma layer was removed
ribs. The pericardium was opened, and the LAD artery was and stored at 20 C until measurements were performed.
ligated using 8-0 silk suture (AD Surgical, Sunnyvale, CA). Plasma blood urea nitrogen (BUN) was subsequently meas-
Occlusion of the LAD artery was visually confirmed by rapid ured using a urea assay kit (BioAssay Systems, Hayward, CA).
myocardial blanching, as well as ST segment elevation on elec-
trocardiogram (ECG) recorded by the PowerLab system Real-Time PCR
(ADInstruments). Then, the ribcage and muscles were closed Total tissue RNA from kidneys was isolated using TRIzol
layer by layer with 7-0 absorbable suture (AD Surgical). The reagent (Invitrogen). SuperScript IV First-Strand Synthesis
skin was closed with 6-0 silk suture (AD Surgical). Sham-oper- System kit (Invitrogen) was used to synthesize cDNA from
ated mice underwent the same procedure without the LAD total RNA from each sample. Quantitative real-time PCR was
artery ligation. Immediately after surgery, mice were admini- performed using TaqMan real-time PCR (7900HT; Applied
stered buprenorphine (0.1 mg/kg sc) for postsurgical analgesia Biosystems). The master mix and all gene probes were also
and subsequently every 8–12 h for 72 h. purchased from Applied Biosystems. The probes used in the
Renal Ischemia-Reperfusion Injury experiments included S18 (Mm02601778), erythropoietin
(Epo; Mm01202755), Slc2a1 (Mm00441480), pyruvate dehy-
Ischemia-reperfusion (IR) AKI was induced as described drogenase kinase (Pdk1; Mm00554300), vascular endothelial
previously (9). Briefly, after being anesthetized with ketamine growth factor (Vegf; Mm00437306), hexokinase 1 (Hk1;
and xylazine (100/10 mg/kg ip), animals underwent right Mm00439344), hexokinase 2 (Hk2; Mm00443385), pyruvate
uninephrectomy followed immediately by left renal pedicle kinase (Pkm1; Mm00834102), phosphoglucomutase (Pgm1;
clamping for 30 min. Mice were warmed on a warming pad Mm00804141), and glyceraldehyde 3-phosphate dehydro-
during the surgery, and reperfusion of the left kidney was genase (Gapdh; Mm99999915).
confirmed after clamp release.
Pimonidazole Staining
Hypoxia Model
Staining was carried out as previously described (13)
Animals were exposed to either room air in our standard using a Hypoxyprobe kit (Burlington, MA). Briefly, 8–
animal facility or to 10% oxygen in a hypoxia chamber for 10 wk after MI, mice received 60 mg/kg pimonidazole
F570 AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
(dissolved in 0.9% saline) via an intraperitoneal injection. ligations were avoided in an attempt to cause cardiac injury
Sixty minutes after injection, animals were euthanized, without a congestive heart failure phenotype (Fig. 1A). When
and kidneys were removed and immediately placed in fix- compared with control mice that underwent a sham proce-
ative containing 3.7% formaldehyde, 10 mM sodium m- dure, this resulted in a small, yet statistically significant,
periodate, 40 mM phosphate buffer, and 1% acetic acid. reduction in cardiac ejection fraction and fractional shorten-
Subsequent processing and immunohistochemical detec- ing as assessed by conscious echocardiography (Fig. 1, B and
tion were performed using the manufacturer-supplied C). Mice that underwent MI did have slightly higher presur-
anti-pimonidazole monoclonal antibody (1:50) according gery body weight, but there was no detectable difference in
to the following immunohistochemistry protocol. The postsurgery weight gain or urine output between the two
M.O.M. immunodetection kit from Vector Laboratories groups (Fig. 1, D–F). In a separate group of animals, GFR was
was used to reduce endogenous mouse IgG staining. not different between the sham and MI groups when meas-
ured 3 wk after surgery (Fig. 1G).
Immunohistochemistry Using this model, we tested the hypothesis that chronic
Following euthanasia, kidneys were removed and incu- cardiac injury worsened renal function following acute is-
bated at room temperature overnight in fixative contain- chemic kidney injury. Nine weeks after cardiac surgery, ani-
ing 3.7% formaldehyde, 10 mM sodium m-periodate, mals that had previously undergone the sham procedure or
40 mM phosphate buffer, and 1% acetic acid. The fixed kid- LAD ligation underwent acute IR injury via renal pedicle
ney was subsequently dehydrated through a graded series clamping for 30 min with contralateral uninephrectomy.
of ethanol, embedded in paraffin, sectioned (4 mm), and Surprisingly, AKI was attenuated in animals that had previ-
mounted on glass slides. Immunostaining was carried out ously received MI, as shown by decreased peak BUN and
as reported previously (14). Sections from both sham and Kim-1 immunostaining following ischemia (Fig. 2, A and B).
MI animals were mounted on a single slide to minimize They also had decreased acute weight loss and mortality
slide-to-slide and temporal variation. Three sections per compared with sham-operated controls that underwent IR
animal were stained to ensure minimal section-to-section (Fig. 2, C and D).
variation. Subsequent images were acquired at the same These data suggested that chronic cardiac injury pro-
time under the same exposure conditions. Quantification moted ischemic preconditioning in the kidney. Previous
was performed using ImageJ software. To do this, thresh- reports have demonstrated that activation of hypoxia-sens-
old parameters were set to differentiate immunopositive ing pathways induces renal ischemic preconditioning (15–
versus negative signal. These parameters were then 17). Thus, we hypothesized that our cardiorenal precondi-
applied to all sections being quantified, and the software tioning might be providing protection via similar mecha-
calculated the percent area that was determined to be pos- nisms. To determine if our MI model caused chronic renal
itive for each image. At least four images were quantified hypoxia, we performed pimonidazole staining on mouse kid-
per animal. Antibodies used include mouse anti-a-smooth neys 2 mo after LAD ligation. This revealed reduced renal ox-
muscle actin (a-SMA; 1:4,000 A5228, Sigma), goat anti-kid- ygen tension in animals that underwent MI compared with
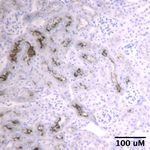
ney injury molecule (KIM)-1 (used for Fig. 2; 1:50 AF1817, sham controls (Fig. 3A and Supplemental Fig. S1; all
R&D), and rat anti-KIM-1 (used for Fig. 6; 1:50 MAB1817- Supplemental Material available online at https://doi.org/
100, R&D). The M.O.M. immunodetection kit (Vector 10.6084/m9.figshare.13519064.v1). As this was highly sugges-
Laboratories) was used to reduce endogenous mouse IgG tive of activated HIF signaling, we measured mRNA levels of
staining in combination with the anti-a-SMA antibody. several HIF target genes. Indeed, mice that had undergone
Negative controls were performed without incubation in MI had increased transcript abundance of Epo, glucose
primary antibody to ensure the absence of nonspecific transporter 1 (Slc2a1), and Pdk1. Vegf mRNA showed a statis-
staining. tical trend toward increased abundance (Fig. 3B).
Renal metabolic reprogramming has been shown to be an
Picrosirius Red Staining important determinant to modulate both acute and chronic
renal injury (15, 16, 18). The HIF pathway is central in this
Staining was performed according to the protocol provided process, by promoting a shift away from oxidative phospho-
by the manufacturer (Sigma, St. Louis, MO). Quantification rylation toward anaerobic glycolysis. To determine if this
was performed using ImageJ software. was occurring in the setting of chronic MI, we measured the
mRNA abundance of key glycolytic enzymes 2 mo after MI,
Statistical Analyses
but before IR. Post-MI mRNA abundance of Hk1, Hk2, Pkm1,
All values are expressed as means ± SE. Between-group Pgm1, and Gapdh were all increased when compared with
comparisons were made using two-sided Student’s t test or sham-operated controls (Fig. 3C).
two-way ANOVA, as indicated in the figure legends. A P These data provided evidence for chronic cardiac ischemia
value ofEFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
A 0 9 13
Time (weeks)
Chronic
Injury
LAD ligation IR AKI + UNx Evaluation
Figure 1. Effects of myocardial infarction (MI)
on cardiac and renal function. A: animals
underwent either sham or left anterior de-
scending artery (LAD) ligation at time 0. Nine
weeks later, animals in both groups under-
B C D Sham
went ischemia-reperfusion acute kidney injury 95 70 MI
(IR AKI) with contralateral uninephrectomy 35
(UNx). Chronic effects on renal injury and fi- 90
60
brosis were subsequently determined at 30
85
13 wk after the initial cardiac procedure. B: car-
FS, %
EF, %
BW, g
diac ejection fraction (EF) and C: fractional 80 50
shortening (FS) 1 wk after sham or MI surgery. 25
D–G: weight gain (D and E), 24-h urine output 75
(F), and glomerular filtration rate (GFR) (G) after 40
20
mice underwent sham or MI surgery. P <
70
0.05 by unpaired t test. For B and C, n = 4 for 0
65 30 0 5 9
sham and n = 13 for MI. For D and E, n = 5 for Sham MI Sham MI
sham and n = 13 for MI. For F, n = 9 for sham time, weeks
and n = 5 for MI. For G, n = 4 for sham and n =
E F G
5 for MI. 40 UVol, µL * g BW-1 * 24 hr 0.10 1400
GFR, µL * min-1 * 100g-1
30 0.08
Weight gain, %
1300
20 0.06
1200
10 0.04
1100
0 0.02
-10 0.00 1000
Sham MI Sham MI Sham MI
setting of chronic systemic hypoxic conditions. After being DISCUSSION
housed in 10% oxygen for 10 days, wild-type animals that had
not undergone any cardiac procedures were protected from Physiological evidence for communication between the
acute renal ischemic injury. Although the protective effects heart and the kidneys has existed for nearly a century (19).
on BUN elevation and weight loss did not reach statistical sig- Patients suffering from chronic heart failure serve as an im-
nificance until 7 and 14 days, respectively, overall, this was a portant clinical example of the renal consequences endured
similar effect, as was previously observed post-MI (Fig. 4, A when this interorgan communication fails. Indeed, heart
and B). Interestingly, the differences in weight and BUN per- and renal failure coexist frequently, and it is clear that the
sisted for several weeks following acute injury, though BUN cardiorenal syndrome is bidirectional, with primary dys-
did not quite reach statistical significance at later time points function in either organ capable of worsening function in
(Fig. 4, C and D). the other. As the rising prevalence of both heart and kidney
Although the studies described thus far focused on AKI, failure results in more hospitalizations and deaths, a critical
effects on chronic renal injury in our model remained unclear. understanding of the intricacies of this bidirectional com-
To determine long-term effects of MI on progression to chronic munication is key to developing novel therapeutics. To date,
kidney injury after ischemic acute renal injury, we compared there are no specific therapies approved for the treatment of
trends in body weight and BUN between sham and MI groups this syndrome.
following IR. Despite worsened acute injury as already Here, we used a model of ischemic cardiomyopathy, the
described, the sham group had recovery of body weight and most common cause of death in the world, and determined
BUN that was similar to the MI group (Fig. 5, A and B). There effects on both acute and chronic renal injury. Importantly,
was also no detectable difference in GFR 4 wk after IR (Fig. 5C). we honed our model to cause ischemic cardiac injury without
Despite the observed protection from acute renal injury, ani- producing an overt heart failure phenotype at baseline. Given
mals that underwent MI had evidence of worsened that we produced only a modest reduction in cardiac ejection
chronic kidney fibrosis, as shown by increased picrosirius fraction, this scenario is most akin to the clinical entity of
red and a-SMA staining (Fig. 6, A and B). In addition, the heart failure with preserved ejection fraction. Our data show
MI group had increased Kim-1 abundance (Fig. 6C). that even modest cardiac damage affects both acute and
F572 AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
A B
200 *
10 *
Sham
BUN, mg * dL-1
150
8
100
% area
6
Figure 2. Effects of myocardial infarction
50 4 (MI) on ischemic acute kidney injury. A–D:
peak blood urea nitrogen (BUN; A), kidney
2 injury molecule (Kim)-1 immunostaining
0
Sham MI (both low-magnification and high-magnifi-
MI
0 cation images included; B), weight loss (C),
Sham MI and mortality (D) following ischemia reper-
fusion (IR) in animals that had undergone ei-
ther a sham or MI surgery 9 wk prior. Kim-1
* immunostaining was performed on kidneys
24 h following IR. P < 0.05 by unpaired
C 0
D 40
t test. For sham and MI groups, respec-
tively, n = 17 and n = 16 in A, n = 5 and n = 6
30 in B, n = 6 and n = 8 in C, and n = 9 and n =
Mortality, %
Weight loss, %
-5
8 in D.
20
-10
10
-15
0
-20
Sham MI Sham MI
chronic kidney physiology following injury. Surprisingly, underwent MI, consistent with a glycolytic shift precon-
chronic heart damage conferred protection from ischemic ditioning the kidney to respond to ischemic injury.
AKI. Protection was accompanied by evidence of tissue hy- Evidence supporting an important role for HIF signaling
poxia and activation of HIF signaling pathways in the kidney. in acute renal injury in endothelial (29), epithelial (30),
Ischemic preconditioning effects observed in this study and myeloid (31) cell types exists. Determining the cell type(s)
are similar to previous reports of preconditioning observed mediating these effects in our model will be the subject of
with remote ischemia or caloric restriction (20–22). These future studies.
reports focused on calorie restriction or ischemia in remote Our proposed mechanism, although consistent with
organs conferring renal preconditioning via mechanisms prior reports, may initially appear to conflict with clinical
functioning at a distance (23, 24), which is in subtle contrast observations that have been made in individuals with is-
to the present study. In our model, it cannot be excluded chemic cardiomyopathy. Clinical data strongly suggest
that the remote cardiac injury may confer some renal protec- that heart failure increases the risk of AKI, rather than
tion, as has been postulated previously via release of endo- protects from it (32). Although the differences between
crine factors (25). However, our data suggest that ischemic our experimental data and patient studies cannot entirely
preconditioning via a mechanism that has effects directly on be elucidated at this time, several key differences should
renal tissue, namely, chronic renal hypoxia leading to meta- be noted.
bolic reprogramming via activation of hypoxia inducible fac- First, our studies were performed on young mice with
tors. Although our mice that underwent MI demonstrate a healthy kidneys at baseline, a substrate that is quite different
similar phenotype to our chronically hypoxic mice following from the encountered clinical scenario involving older indi-
IR and we observed increased HIF-responsive genes, it viduals with multiple underlying comorbidities affecting re-
should be noted these associations are not mechanistically nal function, including diabetes, hypertension, coronary
definitive. artery disease, peripheral vascular disease, atherosclerosis,
HIF effects are pleiotropic, with studies demonstrating and baseline chronic kidney disease, among others. These
its role in promoting metabolic reprograming toward an- baseline differences make it such that many patients that ex-
aerobic glycolysis, oxygen delivery, erythropoiesis, cellu- perience myocardial ischemia already have baseline kidney
lar proliferation, and cell survival (16, 26). Prior work in dysfunction, which may alter outcomes independent of the
humans and animals has shown preischemic HIF activa- mechanism under investigation in this report. Investigating
tion via acute systemic hypoxia (15, 27), prior renal ische- effects of MI in preinjured or aged kidneys would be interest-
mia (28), or HIF stabilization through prolyl hydroxylase ing to study in the future.
inhibition protects against AKI (17). Beneficial effects in Second, our model involves a permanent LAD ligation,
those studies were accompanied by metabolic reprogram- which does not precisely mimic the corresponding clinical
ming toward anaerobic glycolysis. We observed a similar scenario, which frequently involves coronary reperfusion
increase in glycolytic enzyme transcripts in animals that following percutaneous coronary artery interventions.
AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org F573
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
A Sham MI 10
8
Figure 3. Evidence of renal hypoxia and hy-
poxia-inducible factor (HIF) activation 2 mo
% area
after myocardial infarction (MI). A: pimoni- 6
dazole staining as a marker of renal hy-
poxia 8–10 wk after cardiac surgery, and
4
prior to undergoing renal ischemia reperfu-
sion (IR). B: total kidney mRNA abundance,
as determined by quantitative real-time po- 2
lymerase chain reaction (qPCR), of several
HIF-regulated genes, including erythro-
poietin (Epo), glucose transporter 1 (Slc2a1), 0
pyruvate dehydrogenase kinase (Pdk1), Sham MI
and vascular endothelial growth factor B C
(Vegf) as evidence of HIF signaling. C: total 3 10
mRNA abundance, AU
mRNA abundance, AU
kidney mRNA abundance of several glyco-
lytic enzymes, including hexokinase 1 (Hk1), 8
hexokinase 2 (Hk2), pyruvate kinase (Pkm1),
phosphoglucomutase (Pgm1), and glyceralde- 2
hyde 3-phosphate dehydrogenase (Gapdh). 6
The dotted line in B and C represents
normalized sham control transcript abun- 4
dance. P < 0.05, † P = 0.05, and †† P =
1
0.07 all by unpaired t test. For A, n = 8 for
sham and n = 7 for MI. For B and C, n = 8 2
per group.
0
0
k1
Pk 2
Pg 1
G 1
dh
k
m
m
o
t1
k1
gf
H
H
ap
Ep
lu
Ve
Pd
G
This clinical scenario is associated with cardiac ischemia Third, although clinical data indicate patients with heart
and reperfusion injury rather than permanent ischemia, failure have an increased risk of AKI, one of the more com-
which may alter subsequent effects on renal perfusion and monly observed forms of renal injury in this setting is type 1
injury. cardiorenal syndrome where acute cardiac dysfunction
A B
250 10
200
BUN, mg * dL-1
Sham
Weight loss, %
0
150 MI
-10
100
Figure 4. Effects of chronic systemic hypoxia
on ischemic acute kidney injury. A and B: 50 -20
peak blood urea nitrogen (BUN; A) and
weight loss (B) as measured in healthy ani- 0 -30
Day 1 Day 7 Sham MI
mals housed in either normal or hypoxic con-
ditions (10% O2) for 10 days prior to renal
ischemia. C and D: BUN (C) and weight loss
(D) were monitored for several weeks follow- C Normoxic D Normoxic
ing renal ischemia. P < 0.05 by Mann– Hypoxic Hypoxic
Whitney test, † indicates interaction between 200 20
treatment and time is significant for two-way
ANOVA with repeated measures; n = 4–5 per 10
BUN, mg * dL-1
Weight loss, %
150
group.
0
100
-10
50
-20
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 0 2 4 6 8 10 12 14 16 18 20 22 24 26
Time, days Time, days
F574 AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
A B 0
C
150 1500
GFR, µL * min-1 * 100 g-1
Sham
Weight loss, %
BUN, mg * dL-1
MI -5
100 1000
Sham
-10 MI
50 500
0 -15
1 3 5 7 9 11 13 15 17 19 21 23 25 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 0
Time, days Sham MI
Time, days
Figure 5. Effects of myocardial infarction (MI) on chronic renal function. A–C: blood urea nitrogen (BUN; A), weight loss (B), and glomerular filtration rate
(GFR; C) as measured 4 wk after acute renal ischemia in animals that had previously undergone MI or sham procedure. Comparisons were made by
unpaired t test. For A and B, n = 9 for sham and n = 8 for MI. For C, n = 6 for sham and n = 8 for MI.
results in acute renal dysfunction, which is not an ischemic Finally, the clinical data in this area arise from observatio-
renal insult. This is different from our model in that we are nal studies rather than randomized trials. This is an important
modeling effects of chronic cardiac dysfunction on acute re- distinction to note because the lack of randomization makes
nal ischemia. it more difficult to define cause-and-effect relationships.
A Sham MI
B Sham MI
Figure 6. Effects of myocardial infarction (MI) on kid-
ney fibrosis. A–C: picrosirius red (A), a-smooth mus-
cle actin (a-SMA; B), and kidney injury molecule-1
(KIM-1; C) staining 4 wk after acute renal ischemia in
animals that had previously undergone MI and
sham-operated controls. Each panel includes a low-
magnification image and a high-magnification image
in the inset. Scale bar length for the inset = 50 mM.
P < 0.05 by unpaired t test. For A, n = 3 for sham
and 5 for MI. For B, n = 5 for sham and n = 7 for MI.
For C, n = 5 for sham and n = 8 for MI.
C Sham MI
AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org F575
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
Specifically, although patients with heart failure experience 6,427 patients with heart failure and coronary artery disease. J Am
AKI with increased frequency, making it a risk factor for AKI, Coll Cardiol 44: 1587–1592, 2004. doi:10.1016/j.jacc.2004.06.072.
3. Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the
we cannot clearly delineate the cause-and-effect relationship
definition of a complex symbiosis gone wrong. Intensive care Med
that exists between these two entities. Therefore, it remains 34: 957–962, 2008. doi:10.1007/s00134-008-1017-8.
possible that a significant proportion of the increased AKI risk 4. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen
experienced by patients with heart failure can be attributed to DJ, Hillege HL. Renal impairment, worsening renal function, and out-
confounding variables that put them at increased risk of hos- come in patients with heart failure: an updated meta-analysis. Eur
Heart J 35: 455–469, 2014. doi:10.1093/eurheartj/eht386.
pitalizations and results in a greater AKI incidence. 5. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran
Although acute renal injury and weight loss were amelio- JR, Wynne J; ADHERE Scientific Advisory Committee and
rated post-MI, chronic effects were not. These data from the Investigators. High prevalence of renal dysfunction and its impact on
chronic phase of our model reflect our general understand- outcome in 118,465 patients hospitalized with acute decompensated
ing of cardiorenal syndrome, that is, cardiac dysfunction heart failure: a report from the ADHERE database. J Card Fail 13:
422–430, 2007. doi:10.1016/j.cardfail.2007.03.011.
promoting renal dysfunction. They suggest that although 6. Gonzalez RP, Comba PC, Esteban MR, Sanchez JJ, Afonso JH,
chronic cardiac injury may, to an extent, prime the kidney to Perez MD, Rodriguez IM, Diaz BB, Elosua R, Cabrera de LA.
respond more favorably to an acute insult, it has a deleteri- Incidence, mortality and positive predictive value of type 1 cardiore-
ous effect on renal function over time. As our model nal syndrome in acute coronary syndrome. PLoS One 11: e0167166,
2016. doi:10.1371/journal.pone.0167166.
involved chronic cardiac dysfunction promoting renal dys-
7. Shamseddin MK, Parfrey PS. Mechanisms of the cardiorenal syn-
function, it is most analogous to cardiorenal syndrome type dromes. Nat Rev Nephrol 5: 641–649, 2009. doi:10.1038/nrneph.
2. Although prior studies have focused on type 1 cardiorenal 2009.156.
syndrome, our model provides an opportunity to define car- 8. Matsushita K, Saritas T, Eiwaz MB, McClellan N, Coe I, Zhu W,
diorenal signaling in the chronic setting, outside of acute Ferdaus MZ, Sakai LY, McCormick JA, Hutchens MP. The acute
kidney injury to chronic kidney disease transition in a mouse model
hospitalizations, in the physiological setting where patients of acute cardiorenal syndrome emphasizes the role of inflammation.
spend the majority of their time. This information will have Kidney Int 97: 95–105, 2020. doi:10.1016/j.kint.2019.06.022.
important implications for the development of novel 9. Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K,
therapeutics. Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling
mediates recovery from acute kidney injury. J Clin Invest 122: 4519–
4532, 2012. doi:10.1172/JCI60363.
ACKNOWLEDGMENTS 10. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka
PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD,
The authors thank Volker Haase and Hanako Kobayashi for the Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification
assistance with performing hypoxia chamber experiments. They Writing Group, American Society of Echocardiography's
also thank Lin Zhong for assistance with LAD ligations and Guidelines and Standards Committee, European Association of
echocardiography. Echicardiography. Recommendations for chamber quantification:
a report from the American Society of Echocardiography's Guidelines
and Standards Committee and the Chamber Quantification Writing
GRANTS Group, developed in conjunction with the European Association of
Echocardiography, a branch of the European Society of Cardiology. J
This work was supported by the National Institutes of Health Am Soc Echocardiogr 18: 1440–1463, 2005. doi:10.1161/JAHA.117.
Grants DK51265, DK95785, DK62794, DK7569, and P30DK114809 008181.
(to R.C.H. and M.Z.Z.), by Veterans Affairs Merit Award 00507969 11. Scarfe L, Schock-Kusch D, Ressel L, Friedemann J, Shulhevich Y,
(to R.C.H.), and by the Vanderbilt Center for Kidney Disease. Murray P, Wilm B, de Caestecker M. Transdermal measurement of
glomerular filtration rate in mice. J Vis Exp 58520, 2018. doi:10.3791/
58520.
DISCLOSURES 12. Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D,
A.S.T. has a consulting agreement with Ampio Pharmaceuticals. Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann
No conflicts of interest, financial or otherwise, are declared by the J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measure-
ment of renal function in conscious mice. Am J Physiol Renal Physiol
authors.
303: F783–F788, 2012. doi:10.1152/ajprenal.00279.2012.
13. Ow CPC, Ullah MM, Ngo JP, Sayakkarage A, Evans RG. Detection
AUTHOR CONTRIBUTIONS of cellular hypoxia by pimonidazole adduct immunohistochemistry in
kidney disease: methodological pitfalls and their solution. Am J
A.S.T., M.-Z.Z., and R.C.H. conceived and designed research; Physiol Renal Physiol 317: F322–F332, 2019. doi:10.1152/ajprenal.
A.S.T., K.S., A.N., S.W., X.F., Y.Z., and S.N. performed experiments; 00219.2019.
A.S.T., M.-Z.Z., and R.C.H analyzed data; A.S.T., K.S., J.P.A. M.-Z.Z., 14. Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S,
and R.C.H interpreted results of experiments; A.S.T. prepared fig- Harris RC. Intrarenal dopamine deficiency leads to hypertension
ures; A.S.T. and R.C.H. drafted manuscript; A.S.T., M.-Z.Z., and and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011.
R.C.H edited and revised manuscript; A.S.T., K.S., J.P.A., A.N., doi:10.1172/JCI57324.
S.W., X.F., Y.Z., S.N., M.-Z.Z., and R.C.H approved final version of 15. Johnsen M, Kubacki T, Yeroslaviz A, Spath MR, Morsdorf J, Gobel
H, Bohl K, Ignarski M, Meharg C, Habermann B, Altmuller J, Beyer
manuscript. A, Benzing T, Schermer B, Burst V, Muller RU. The integrated RNA
landscape of renal preconditioning against ischemia-reperfusion
REFERENCES injury. J Am Soc Nephrol 31: 716–730, 2020. doi:10.1681/ASN.
2019050534.
1. WHO. The Top 10 Causes of Death. World Health Organization. 16. Kapitsinou PP, Haase VH. Molecular mechanisms of ischemic pre-
https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes- conditioning in the kidney. Am J Physiol Renal Physiol 309: F821–
of-death. [2020 Dec 9]. F834, 2015. doi:10.1152/ajprenal.00224.2015.
2. Ezekowitz J, McAlister FA, Humphries KH, Norris CM, Tonelli M, 17. Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-
Ghali WA, Knudtson ML; APPROACH Investigators. The associa- Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxyla-
tion among renal insufficiency, pharmacotherapy, and outcomes in tion inhibits fibrosis associated with acute kidney injury. Am J
F576 AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.EFFECTS OF MYOCARDIAL INFARCTION ON KIDNEY INJURY
Physiol Renal Physiol 302: F1172–F1179, 2012. doi:10.1152/ajprenal. risk patients undergoing cardiac surgery: a randomized clinical trial.
00667.2011. JAMA 313: 2133–2141, 2015. doi:10.1001/jama.2015.4189.
18. Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao 25. Wakasaki R, Matsushita K, Golgotiu K, Anderson S, Eiwaz MB,
J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Orton DJ, Han SJ, Lee HT, Smith RD, Rodland KD, Piehowski PD,
Defective fatty acid oxidation in renal tubular epithelial cells has a Hutchens MP. Glomerular filtrate proteins in acute cardiorenal syn-
key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. drome. JCI Insight 4: e122130, 2019. doi:10.1172/jci.insight.122130.
doi:10.1038/nm.3762. 26. Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance
19. Merrill AJ, Morrison JL, Branno ES. Concentration of renin in renal to kidney disease. Kidney Int 69: 1302–1307, 2006. doi:10.1038/sj.
venous blood in patients with chronic heart failure. Am J Med 1: 468, ki.5000221.
1946. doi:10.1016/0002-9343(46)90067-8. 27. Vesnina ZV, Lishmanov YB, Alexandrova EA, Nesterov EA.
20. Grundmann F, Muller RU, Reppenhorst A, Hulswitt L, Spath MR, Evaluation of nephroprotective efficacy of hypoxic preconditioning
Kubacki T, Scherner M, Faust M, Becker I, Wahlers T, Schermer B, in patients undergoing coronary artery bypass surgery. Cardiorenal
Benzing T, Burst V. Preoperative short-term calorie restriction for Med 6: 328–336, 2016. doi:10.1159/000446571.
prevention of acute kidney injury after cardiac surgery: a random- 28. Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells
ized, controlled, open-label, pilot trial. J Am Heart Assoc 7: e008181, contribute to the protective effect of ischemic preconditioning in the
2018. doi:10.1161/JAHA.117.008181. kidney. Kidney Int 77: 771–780, 2010. doi:10.1038/ki.2010.12.
21. Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van 29. Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian
den Engel S, Chu T, Forrer F, Muller C, de Jong M, van IW, Jn IJ, A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL,
Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and Sutton TA, Haase VH. Endothelial HIF-2 mediates protection and re-
fasting precondition against ischemia reperfusion injury in mice. covery from ischemic kidney injury. J Clin Invest 124: 2396–2409,
Aging Cell 9: 40–53, 2010. doi:10.1111/j.1474-9726.2009.00532.x. 2014. doi:10.1172/JCI69073.
22. Spath MR, Bartram MP, Palacio-Escat N, Hoyer KJR, Debes C, 30. Schley G, Klanke B, Schodel J, Forstreuter F, Shukla D, Kurtz A,
Demir F, Schroeter CB, Mandel AM, Grundmann F, Ciarimboli G, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam
Beyer A, Kizhakkedathu JN, Brodesser S, Gobel H, Becker JU, C. Hypoxia-inducible transcription factors stabilization in the thick
Benzing T, Schermer B, Hohne M, Burst V, Saez-Rodriguez J, ascending limb protects against ischemic acute kidney injury. J Am
Huesgen PF, Muller RU, Rinschen MM. The proteome microenvir- Soc Nephrol 22: 2004–2015, 2011. doi:10.1681/ASN.2010121249.
onment determines the protective effect of preconditioning in cispla- 31. Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J,
tin-induced acute kidney injury. Kidney Int 95: 333–349, 2019. Rivella S, Schlondorff D, Haase VH. Myeloid cell-derived hypoxia-
doi:10.1016/j.kint.2018.08.037. inducible factor attenuates inflammation in unilateral ureteral
23. Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, obstruction-induced kidney injury. J Immunol 188: 5106–5115, 2012.
Klibanov AL, Kalantari K, Rosin DL, Okusa MD. Ultrasound prevents doi:10.4049/jimmunol.1103377.
renal ischemia-reperfusion injury by stimulating the splenic choliner- 32. Jentzer JC, Bihorac A, Brusca SB, Del R-PG, Kashani K, Kazory A,
gic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, Kellum JA, Mao M, Moriyama B, Morrow DA, Patel HN, Rali AS,
2013. doi:10.1681/ASN.2013010084. van Diepen S, Solomon MA; Critical Care Cardiology Working
24. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn Group of the Heart Failure and Transplant Section Leadership
PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Council. Contemporary management of severe acute kidney injury
Gorlich D, Kellum JA, Meersch M; RenalRIPC Investigators. Effect and refractory cardiorenal syndrome: JACC council perspectives. J
of remote ischemic preconditioning on kidney injury among high- Am Coll Cardiol 76: 1084–1101, 2020. doi:10.1016/j.jacc.2020.06.070.
AJP-Renal Physiol doi:10.1152/ajprenal.00476.2020 www.ajprenal.org F577
Downloaded from journals.physiology.org/journal/ajprenal (035.134.136.242) on May 2, 2021.You can also read